
In-Depth Report
Welcome to ASH 2021
-
The 63rd ASH Annual Meeting took place as a hybrid event on 11th–14th December 2021, with attendees able to either attend the event in person in Atlanta, Georgia, USA, or via a virtual congress platform. As the world’s most comprehensive malignant and non-malignant haematology event of the year, the meeting continued its tradition of providing an invaluable educational experience and the opportunity to access updates in the hottest topics in haematology. The mission of ASH is to further the understanding, diagnosis, treatment, and prevention of disorders affecting the blood, bone marrow, and the immunologic, haemostatic, and vascular systems, by promoting research, clinical care, education, training, and advocacy in haematology.

Lymphoma
Genetics
-

James R. Cerhan, Mayo Clinic, Rochester, USA, discussed the role of inherited variants which have the potential to cause lymphoma. Genetic predisposition is an important factor in the pathogenesis of lymphoma with evidence of both rare and common inherited variants, even for risk of developing the monoclonal B-cell lymphocytosis precursor condition. B-cell and T-cell neoplasias appear common in individuals with primary immunodeficiencies and immune dysregulatory disorders (i.e. inborn errors in immunity), with more than 300 single gene defects affecting the immune system. In addition, viral infections, such as Epstein Barr virus, have been shown to increase susceptibility to developing cancer.
Of note, while there are suggestive data linking rare and low-frequency variants in selected immune and cancer susceptibility genes with lymphoma, there are no strong lymphoma subtype associations. However, GWAS have provided “a foothold into the genome” and provided a genetic underpinning to familial risk.
“GWAS implicate dysregulation of immunity genes in CLL.”
- James R. Cerhan, Mayo Clinic, Rochester, USA
Sandrine Roulland, Centre d’Immunologie de Marseille Luminy, Marseille, France, discussed the role of early lesions in the development of follicular lymphoma (FL). While FL is exquisitely responsive to immunochemotherapy, many patients follow a relapsing remitting clinical course driven in part by a common precursor cell population. While a (14;18) translocation [t(14;18)] constitutes both a genetic hallmark and critical early event in the natural history of FL, it is also detectable in the blood of otherwise healthy individuals. Clonal analysis of t(14;18) junctions in paired pre-diagnostic blood versus tumour samples have demonstrated that progression to FL can occur from t(14;18)-positive committed precursors. Furthermore, healthy participants at enrolment who develop FL up to 15 years later show a markedly higher t(14;18) prevalence and frequency than controls, and have a 23-fold higher risk of subsequent FL. Several additional mutations have also been identified in t(14;18) pre-diagnostic samples including recurrent mutations of the histone modifier CREBBP.
“High t(14;18) frequency in blood from healthy individuals defines the first predictive biomarker for FL, effective years before diagnosis.”
- Sandrine Roulland, Centre d’Immunologie de Marseille Luminy, Marseille, France
Indolent lymphomas
-
Patients with indolent lymphoma share a long disease course, with current treatment paradigms aiming to prolong quality of life and duration of treatment response. The ability to provide personalised care for patients with indolent lymphoma requires a careful approach that balances individual patient clinical, biologic, and genetic considerations towards the understanding of assessing patient risk.
Carla Casulo, James P. Wilmot Cancer Institute, Rochester, USA, discussed patient and tumour-specific factors associated with risk of disease progression, histologic transformation, and premature death from FL. While at present, there is no one agreed, uniform definition of risk in FL, disease survival data spanning over two decades and multiple available novel therapeutics with high response rates and long periods of remission provide a reasonable benchmark of expected patient outcomes at diagnosis. Despite a favourable prognosis for most patients, subsets with a ‘high-risk phenotype’ can have poor clinical outcomes due to early progression, multiple relapsed/refractory disease, and/or histologic transformation to aggressive lymphoma. A number of clinical prognostic tools and risk scores for FL are available.
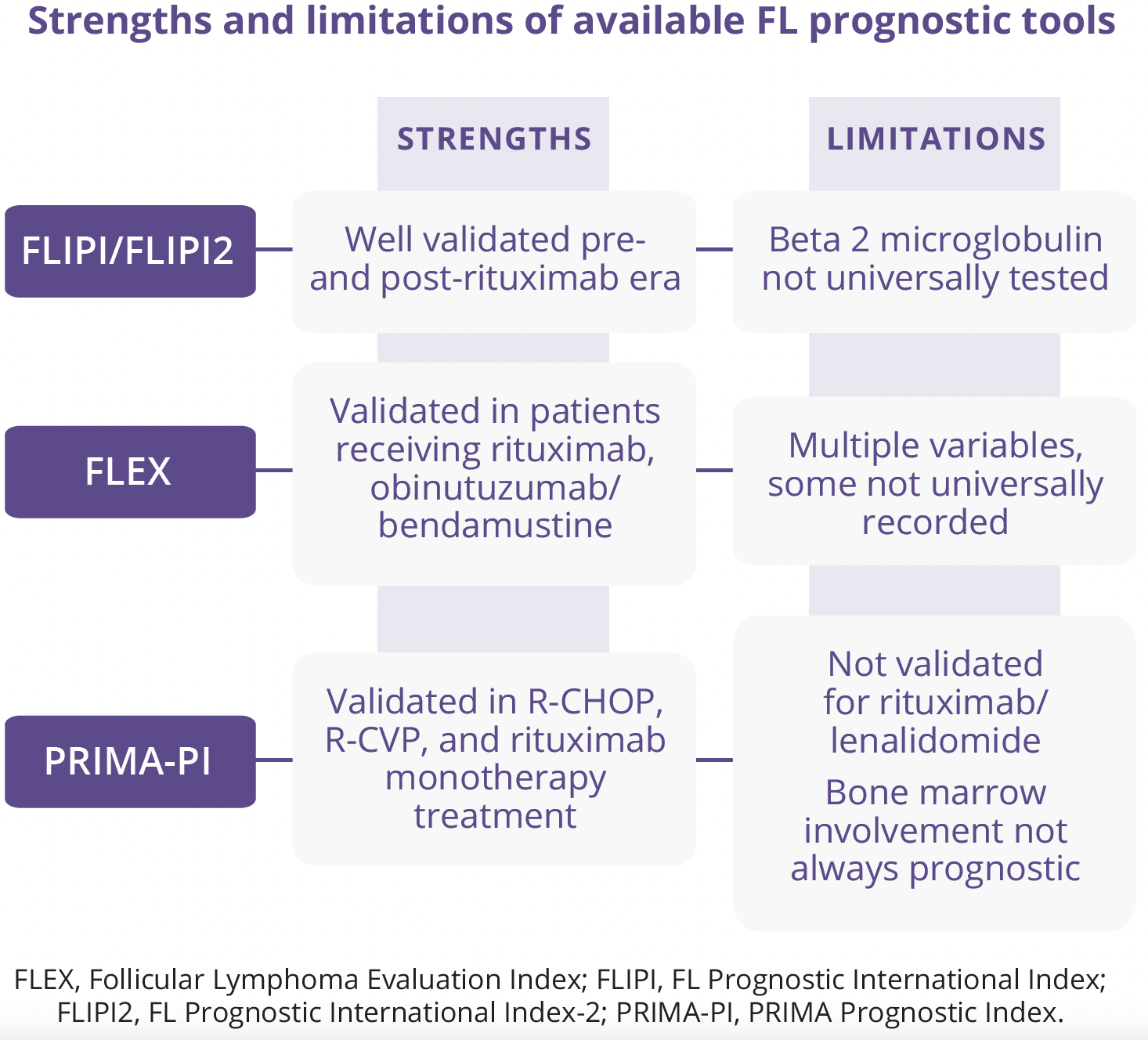
The combination of seven key epigenetic mutations with a high risk FL Prognostic International Index score and patient performance status has yielded the m7-FLIPI. However, while this improved risk stratification in patients with advanced stage FL receiving R-CHOP and R-CVP, it was not predictive in patients receiving bendamustine/obinutuzumab or rituximab/interferon.

Gene expression profile studies have established an association between the tumour microenvironment (TME) and FL survival. The Bio-clinical FLIPI has integrated the FLIPI with genes implicated in the TME and identified several associated with early treatment failure, although only lack of intrafollicular CD4 expression was predictive.
There are currently a number of areas of unmet need in the risk assessment in FL:
- • Studies of risk-adapted therapy based on circulating markers
- • Dynamic risk assessment merging clinical and biologic data over time
- • Risk models agnostic of therapy/validated with multiple treatments
- • Risk stratification at recurrence
“To date, no risk model has been validated to support treatment selection or adaption.”
- Carla Casulo, James P. Wilmot Cancer Institute, Rochester, USA
Robin Foà, Sapienza University, Rome, Italy, explored the potential role of minimal residual disease (MRD) in the management of patients with indolent non-Hodgkin’s lymphoma (NHL), focusing on FL.
Although studies investigating the possible role of MRD monitoring in FL go back more than two decades, MRD is still not in the recommendations/guidelines for the day-to-day management of patients with FL (and in general for indolent NHL). The situation in FL is reminiscent of that currently in place in chronic lymphocytic leukaemia (CLL). In CLL, MRD can be monitored virtually in all patients (not like in FL) using flow cytometry and real-time quantitative polymerase chain reaction. MRD negativity has been associated in CLL with better survival rates and even cure. Nonetheless, MRD monitoring is still not recommend for the daily management of patients with CLL outside of clinical trials. In both advanced and localised FL, quantification of molecular tumour burden at baseline predicts progressionfree survival (PFS), being an independent prognostic marker. Circulating B-cell lymphoma-2 (BCL2)/immunoglobulin heavy chain levels, which may simply reflect not only tumour burden but also the enhanced lymphoma cell migration and invasiveness, could also help to refine the capacity to risk stratify patients. MRD can be used to refine clinical response. MRD negativity is predictive of a better PFS in all clinical trials conducted in the last two decades, with and without maintenance, even in relapsed patients, and possibly of a longer survival in studies with a prolonged follow up.
Assessment of MRD at earlier time points compared with end of induction can also be informative. Patients with FL and a higher relapse probability can be identified, but the timing of clinical relapse is not accurately predicted by current MRD analyses. However, a kinetic model of MRD analysis, more than a punctual single result, could help in anticipating disease recurrence. Can MRD be used to modulate treatment? Available study data suggest one single molecular response evaluation at the end of induction is probably not sufficient to indicate disease eradication and to de-intensify treatment. In order to move MRD in FL from clinical trials to daily clinical practice, further studies are needed.
“The combination of molecular and metabolic response assessment is a promising and valuable tool to be further explored.”
- Robin Foà, Sapienza University, Rome, Italy
Aggressive lymphomas
-
Kieron Dunleavy, Lombardi Cancer Center, Washington, USA, explored up-front and later therapy selection in ‘double-hit’ and other high-grade B-cell lymphomas (HGBL). While the outcome for many patients with newly diagnosed aggressive B-cell lymphoma is positive, several subtypes, such as HGBL, can be therapeutically challenging. The high-grade morphology of these lymphomas and frequently harboured MYC and BCL2 and/or BCL6 translocations has led to their separate categorisation and distinction from diffuse large B-cell lymphoma (DLBCL). Most HGBLs are characterised by distinct rearrangements, although others can have high-grade morphological features without these rearrangements and are known as HGBL-not otherwise specified. Available data suggest HGBLs typically have poor clinical outcomes following standard rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone therapy. Retrospective and recent single-arm, multicentre studies suggest management with dose-intense treatment platforms may be more appropriate, although this therapeutic approach has yet to be validated in randomised study settings. In the relapsed and refractory setting, novel approaches such as anti-CD19 chimeric antigen receptor T (CAR-T) cells and antibodies against CD19 have demonstrated high efficacy in patients with HGBLs. Recent genomic studies have provided insights into some of the molecular pathways that drive lymphomagenesis to allow testing for additional novel therapeutic approaches.
“Management of DLBCL remains a huge therapeutic challenge.”
- Kieron Dunleavy, Lombardi Cancer Center, Washington, USA
Björn Chapuy, Charité, Berlin, Germany, explored recent advances in the understanding of the molecular heterogeneity of DLBCL to support the development of new therapies. DLBCL is a genetically heterogeneous disease with multiple genetic subtypes, such as transcriptionally defined activated B-cell (ABC) and germinal center B-cell (GCB), which have been validated using targeted approaches. Genetically-defined DLBCL subsets (C1–C5) predict different outcomes, provide novel insights into lymphomagenesis, and suggest certain combinations of targeted therapies. Co-targeting of phosphoinositide 3-kinase (PI3K) (e.g. with copanlisib) and BCL2 (e.g. with venetoclax) is highly synergistic in genetically-defined preclinical DLBCL models (e.g. C3 DLBCL) and provides a roadmap for rational pre-clinical therapies. However, R-CHOP continues to have a role in the treatment of DLBCL. Available data suggest that R-CHOP in combination with other newer agents (e.g. use of the Bruton tyrosine kinase inhibitor ibrutinib in the Phoenix study [NCT01855750]) is effective in young patients with activated B-cell DLBCL.

Understanding molecular vulnerabilities in select lymphoid subtypes and integrating this information in future trial design provides the roadmap to precision lymphoma treatment with the hope to improve prospects of patients with unfavourable prognosis.
“R-CHOP-like is a gold standard for more than 20 years and sets a very high bar in terms of trial design for future all-comer studies.”
- Björn Chapuy, Charité, Berlin, Germany
Reem Karmali, Northwestern University, Chicago, USA, outlined the strengths and limitations of CD19 CAR-T cells as customised engineered products versus off-the-shelf immunotherapies, such as bispecific CD20-CD3 antibodies, as therapeutic options for relapsed/refractory aggressive B-cell NHLs. Three customised CD19-engineered CAR-T cell constructs are now available for use as third-line therapies and beyond for aggressive B-cell NHL. Therapeutic responses with CAR-T cells are typically durable in 30–40% of patients globally, with consistent results reported in older patients, primary refractory disease, HGBL, and patients with concurrent secondary central nervous system disease, all features historically associated with poorer outcomes. There are a number of challenges associated with the use of CAR-T cells.
Rapid development of ‘off-the-shelf’ immunotherapies – CD20/CD3 bispecific antibodies – have shown promising activity in aggressive B-NHL, with the potential to circumvent some of the challenges encountered with CAR-T cells, although further studies and longer-term follow-up are needed to elucidate optimal sequencing.
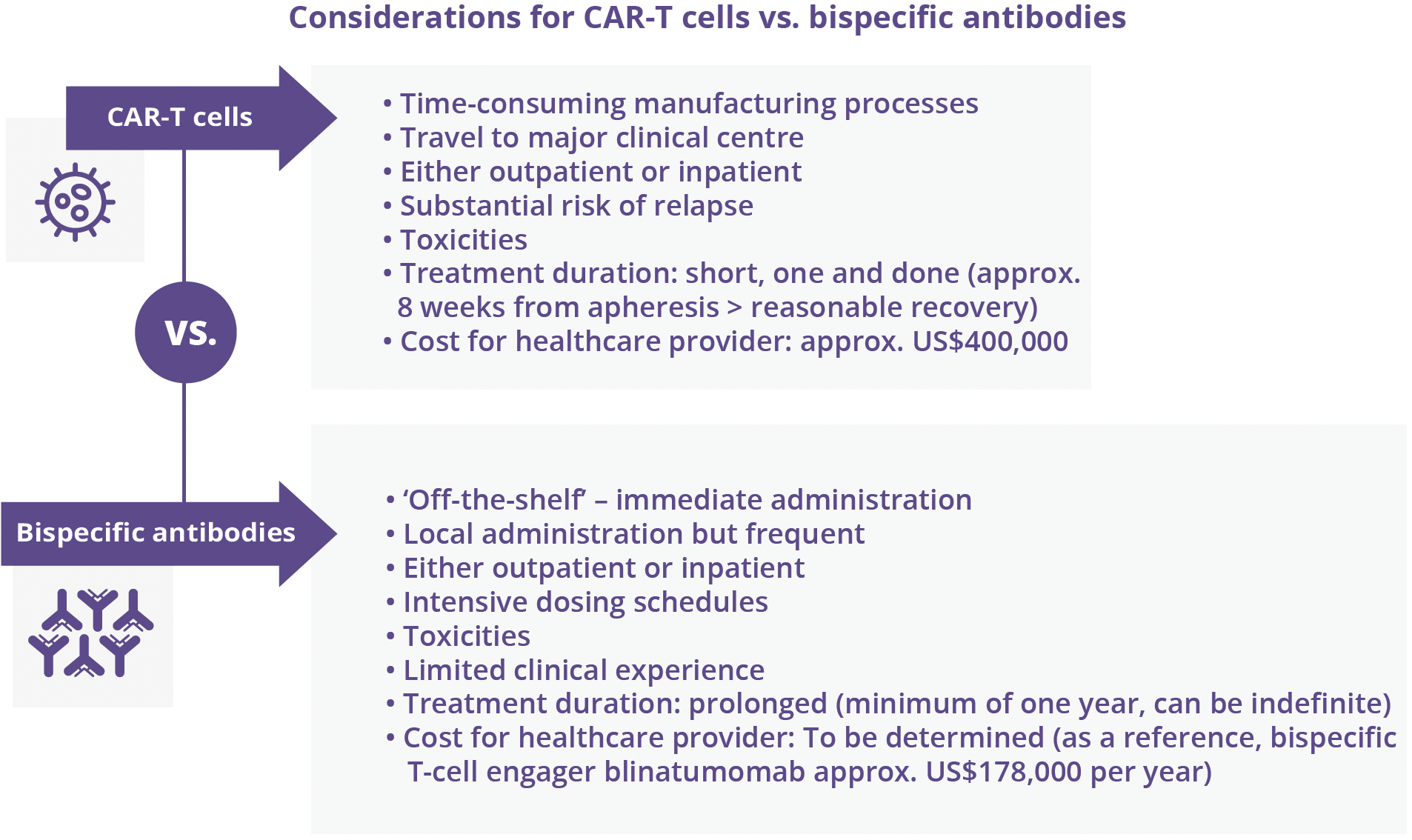
“Ongoing experience with both customised engineered and off-the-shelf immunotherapies will provide guidance on optimal methods of delivery and sequencing.”
- Reem Karmali, Northwestern University, Chicago, USA
Closing remarks
-
ASH 2021 provided an invaluable educational experience, with attendees able to access new haematology data and insights, both virtually and in person, which will hopefully stimulate new ways of thinking to support optimal patient care. The revised hybrid format for 2021 continued to provide the opportunity for participants, particularly those outside the US, to attend ASH perhaps for the first time, enabling more institutions around the world to gain access to the meeting content. The meeting in Atlanta, Georgia, USA, also allowed peer-to-peer interaction and the chance to interact face-to-face with top minds in the field.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.
Sign up or login to unlock the full suite of MEDICALLY features