
In-Depth Report
Welcome to ASH 2021
-
The 63rd ASH Annual Meeting took place as a hybrid event on 11th–14th December 2021, with attendees able to either attend the event in person in Atlanta, Georgia, USA, or via virtual congress platform. As the world’s most comprehensive malignant and non-malignant haematology event of the year, the meeting continued its tradition of providing an invaluable educational experience and the opportunity to access updates in the hottest topics in haematology. The mission of ASH is to further the understanding, diagnosis, treatment, and prevention of disorders affecting the blood, bone marrow, and the immunologic, haemostatic, and vascular systems, by promoting research, clinical care, education, training, and advocacy in haematology.

Leukaemia
Genetics
-

Geffen Kleinstern, University of Haifa, Israel, discussed the role of inherited variants with risk of monoclonal B-cell lymphocytosis (MBL), the precursor to chronic lymphocytic leukaemia (CLL). Next generation sequencing has identified approximately 60 genes recurrently mutated in patients with CLL. The additive prognostic value of the total number of recurrently mutated CLL genes (i.e. tumour mutational load [TML]) or the individually mutated genes beyond the CLL international prognostic index (CLLIPI) in newly diagnosed CLL and high-count MBL has been investigated. Available data suggest that TML is a strong prognostic factor for time to first treatment independent of CLL-IPI, particularly among low/intermediate CLL-IPI risk, and a better predictor than any single gene.

“Mutational screening at early stages may improve risk stratification and better predict time to first treatment.”
- Geffen Kleinstern, University of Haifa, Haifa, Israel
Chronic lymphocytic leukaemia
-
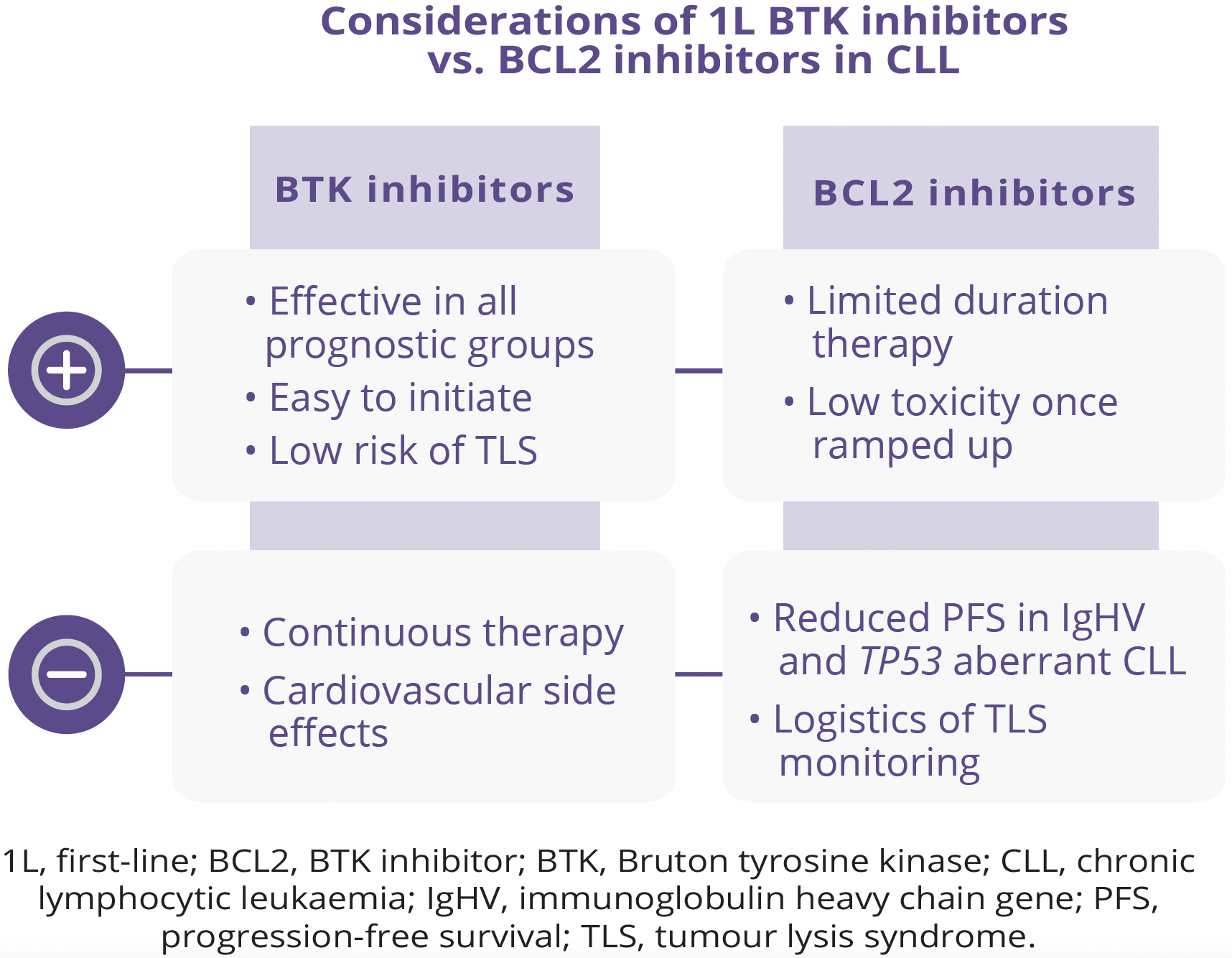
The availability of small molecule therapy with or without antibodies for the treatment of CLL has dramatically prolonged patient survival compared with that expected with traditionally used chemoimmunotherapy-based therapies. However, it remains unclear whether continuous or finite therapy regimens are optimal approaches. In addition, ideal sequencing of small molecule therapy has been unclear and there may not be one best strategy that fits all patients. Constantine S. Tam, Royal Melbourne Hospital, Melbourne, Australia, discussed the benefits and limitations of continuous therapy in patients with CLL. Both B-cell receptor (BCR) inhibitors and the B-cell lymphoma-2 (BCL2) inhibitor venetoclax have been utilised in continuous therapy regimens. While both Bruton tyrosine kinase (BTK) inhibitors and BCL2 inhibitors are regarded as standards of care for first-line (1L) treatment of CLL, there are several arguments for favouring BTK inhibitors as 1L therapy in CLL, including superior progression-free survival (PFS) across multiple comparisons with chemotherapy. However, one limitation of BTK inhibitors is that they cannot clear minimal residual disease (MRD), hence the requirement for indefinite use. In addition, del(17p) and TP53-mutated CLL appears to be prognostically important given the limited PFS benefit seen with the use of ibrutinib as monotherapy or combined with rituximab in TP53 aberrant CLL. Similarly, although venetoclax-based regimens have the advantage of being fixed in duration, patients with highrisk features may experience inferior PFS relative to those without these features.
“Next-generation BTK inhibitors, such as acalabritinib and zanubrutinib, are associated with reduced toxicities in head-to-head comparisons with ibrutinib.”
- Constantine S. Tam, Royal Melbourne Hospital, Melbourne, Australia
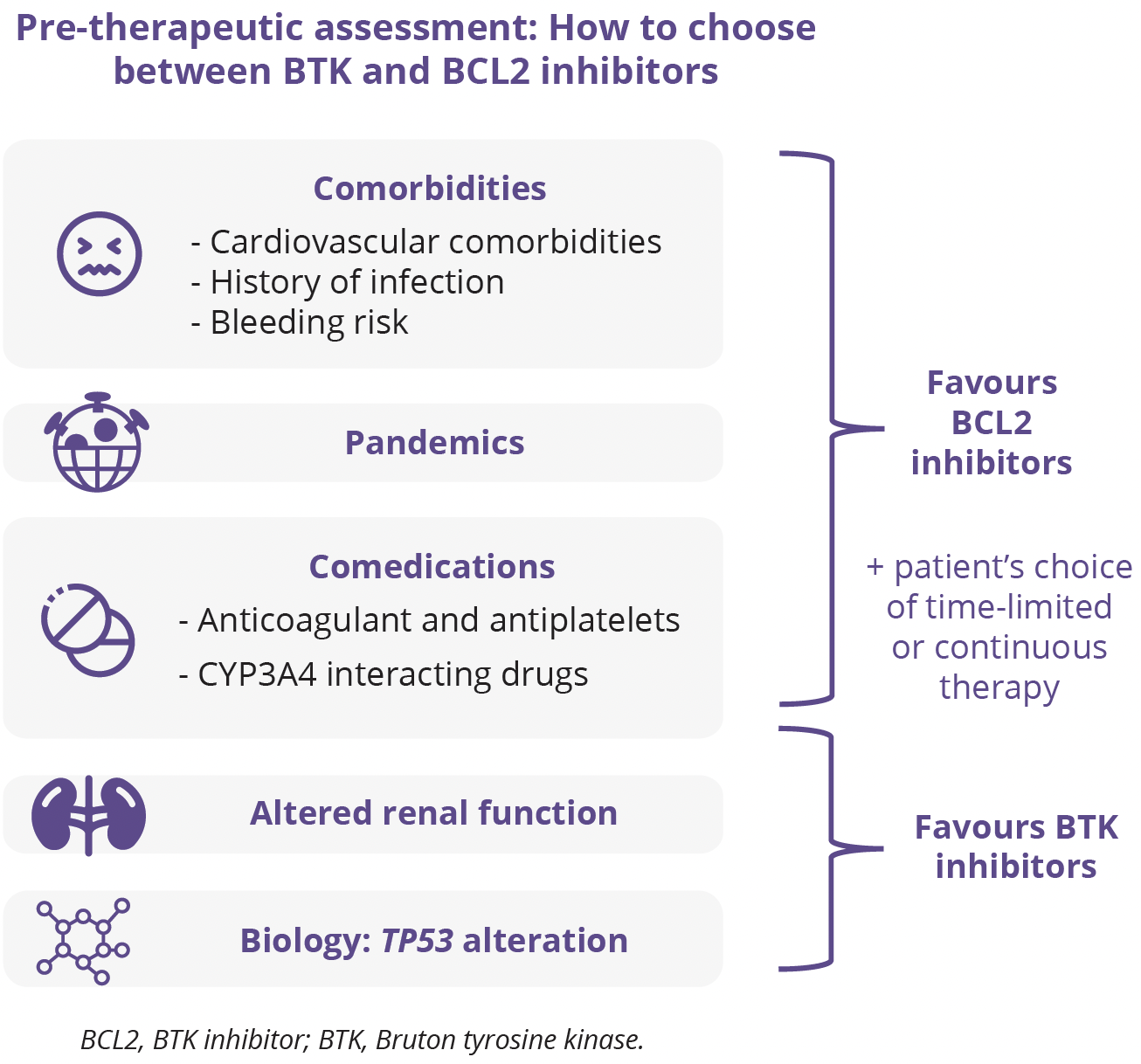
Florence Cymbalista, Hôpital Avicenne, Paris, France, discussed the opportunities for and challenges of time-limited 1L treatment in CLL. Until the arrival of BCR inhibitors, treatment mainly relied on chemotherapy and was therefore of limited duration. In recent years, the use of BTK inhibitors has expanded into frontline therapy, although being given continuously until progression has partly erased the aim of reaching undetectable MRD. Conversely, venetoclax either as monotherapy or in combination with other agents can be discontinued safely after an optimal treatment response is achieved. Thus, the use of time-limited treatment is now increasing, with the goal of reaching deep MRD responses. Combining targeted agents is very promising, but it is important to keep in mind that follow-up is still short and that information on the efficacy of subsequent lines of therapy is currently lacking. Sensitive MRD should help tailor the optimal duration of the various strategies. The rapidly increasing understanding of genetic background and clonal evolution is not yet integrated into therapeutic decisions, but in the coming years it could replace the ‘one-size-fits-all’ tendency and allow more personalised treatment approaches.
“Numerous ongoing studies demonstrate the aspiration of both physicians and patients for time-limited therapies.”
- Florence Cymbalista, Hôpital Avicenne, Paris, France
Anti-CD20 monoclonal antibodies (mAbs) have revolutionised the treatment of CLL by improving survival of patients with CLL in conjunction with chemotherapy. However, the novel targeted agents such as BTK inhibitors and venetoclax have now mostly replaced chemotherapy in 1L treatment of CLL. Deborah Stephens, University of Utah, Salt Lake City, USA, explored the use of anti-CD20 mAb combination therapies in patients with CLL. Several clinical studies have assessed the role of anti-CD20 mAbs in combination with BTK inhibitors and venetoclax. Of note, the addition of rituximab to ibrutinib does not improve PFS in treatment-naïve patients with CLL, which may be related to ibrutinib’s antagonistic effect on anti-CD20 antibodies. In contrast, the addition of the glycoengineered anti-CD20 mAb obinutuzumab to the more selective BTK inhibitor acalabrutinib may improve PFS, although does not appear to improve overall survival (OS) of patients with CLL in the 1L setting (OS data not yet mature). In contrast to BTK inhibitors, a combination of fixed-duration venetoclax with an anti-CD20 mAb can induce deep remission with high rates of undetectable MRD, correlating with improved survival in patients with CLL in both 1L and relapsed/ refractory settings.
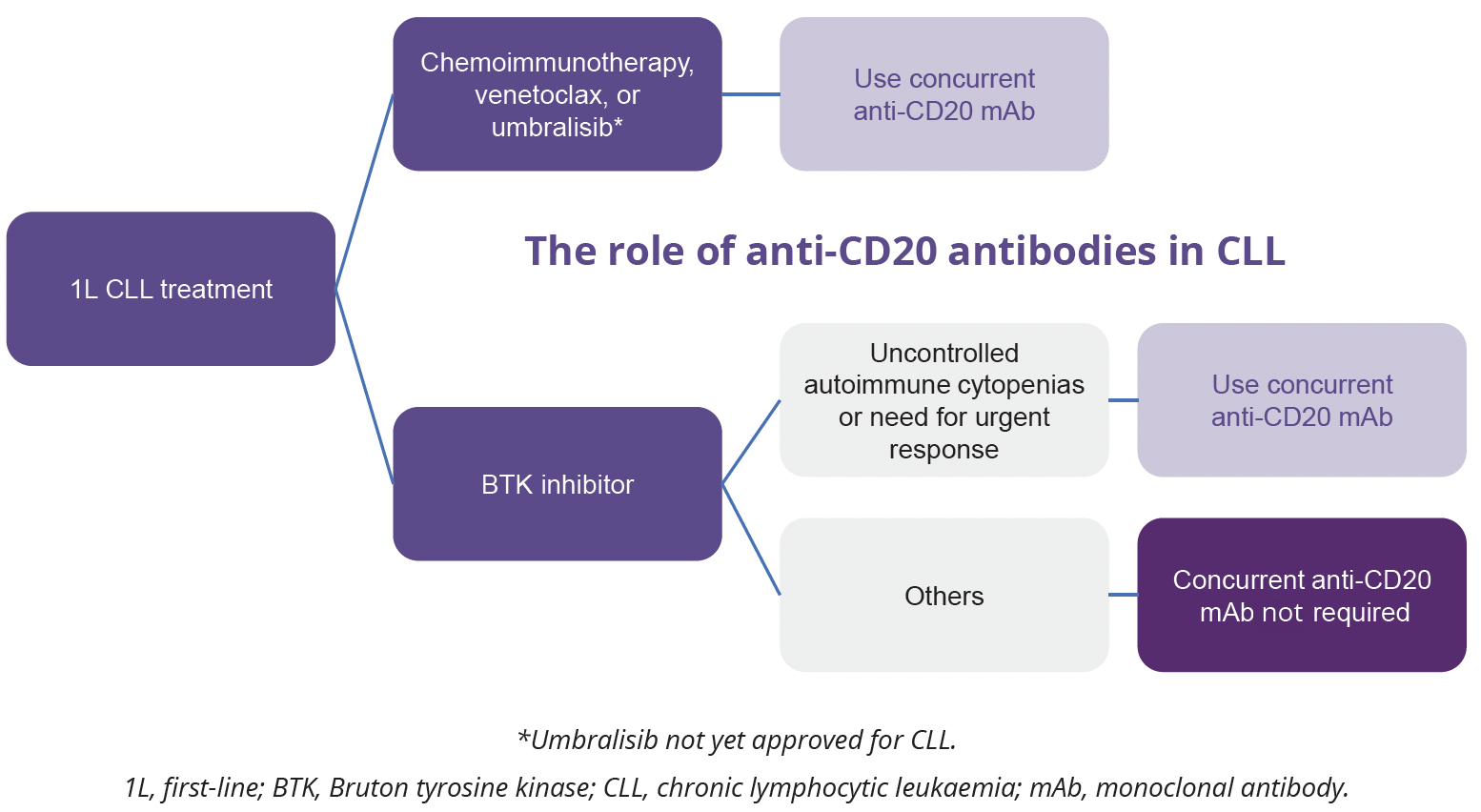
“The addition of an anti-CD20 mAb to a BTK inhibitor appears to provide most therapeutic benefit in patients with autoimmune cytopenia or rapidly progressive disease.”
- Deborah Stephens, University of Utah, Salt Lake City, USA
Closing remarks
-
ASH 2021 provided an invaluable educational experience, with attendees able to access new haematology data and insights, both virtually and in person, which will hopefully stimulate new ways of thinking to support optimal patient care. The revised hybrid format for 2021 continued to provide the opportunity for participants, particularly those outside the US, to attend ASH perhaps for the first time, enabling more institutions around the world to gain access to the meeting content. The meeting in Atlanta, Georgia, USA, also allowed peer-to-peer interaction and the chance to interact face-to-face with top minds in the field.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.