
Welcome to ESMO 2022
-
The Annual Meeting of the European Society of Medical Oncology (ESMO) was a hybrid meeting, with delegates attending in person for the first time since before the pandemic. ESMO President Solange Peters noted that this made the meeting “feel like a huge success before it has even begun” as the oncology community were able to meet, network and disseminate data in-person in Paris, France. She underlined that, for ESMO, there was never going to be a return to “normal” but was instead progressing forward due to the proactivity that the community has demonstrated in reinventing itself to meet the challenges of our era.

New cancer cases predicted globally by 2030 and 2040
Globally, the ESMO community is already taking action by going the extra mile to comfort patients, to grow as professionals by keeping their knowledge up-to-date, and by raising awareness for cancer prevention. The ESMO community is driven by a shared determination to work towards the best possible outcomes for patients with an inspiring tagline of, ‘Together we can. Together we care.’
Solange Peters then summarised the importance of healthcare sustainability and how our healthcare systems rely on finite resources. She emphasised that a key responsibility of the oncology community should be safeguarding patients’ ability to access high-quality care. ESMO should continue to contribute to the overall sustainability of the healthcare system by nurturing the professional development of oncologists and offering continued support in their day-to-day clinical practice. ESMO’s support and resources should therefore reach every oncology professional.
“As important as doctors’ knowledge and skill is their physical and mental ability to give the best of themselves to patients each day.”
- Solange Peters, ESMO President
Solange Peters then introduced the ESMO 2022 Scientific Co-Chairs, Fabrice André and Charles Swanton. Charles Swanton gave an overview of the impressive proportion of delegates attending ESMO 2022 both in-person and online. He then stated that the purpose of ESMO 2022 is not only a “celebration of getting us all back together”, but also a collaboration between diverse oncology professionals who spend their lives aiming to improving the survival of their patients. ESMO 2022 will focus on “better understanding the disease and treating patients better – from bench to bedside”, which is a matter of time, collaboration, patience and long-term investment. Fabrice André added that current challenges in oncology will be integrated within the ESMO Congress 2022 programme such as early cancer detection and prevention, molecular medicine, and the impact of new drugs assessed in underrepresented patients such as anti-programmed cell death protein-1 (PD1) in the elderly population.

Total participants online and in person
Who should I test for genetics outside of BRCA?
In this multidisciplinary session the question of genetic testing outside of BRCA was discussed from a surgical, medical oncology and genetic counsel point of view.
What and who to test beyond BRCA
In his talk, Dr Kevin Punie, Leuven, Belgium, emphasised the importance of genetic testing beyond BRCA as there is a plethora of hereditary breast cancer genes with BRCA1 and BRCA2 variants making up only one fifth of hereditary breast cancer cases.
He warned that there are important implications from hereditary dispositions as these genes not only influence patients but can affect relatives too.
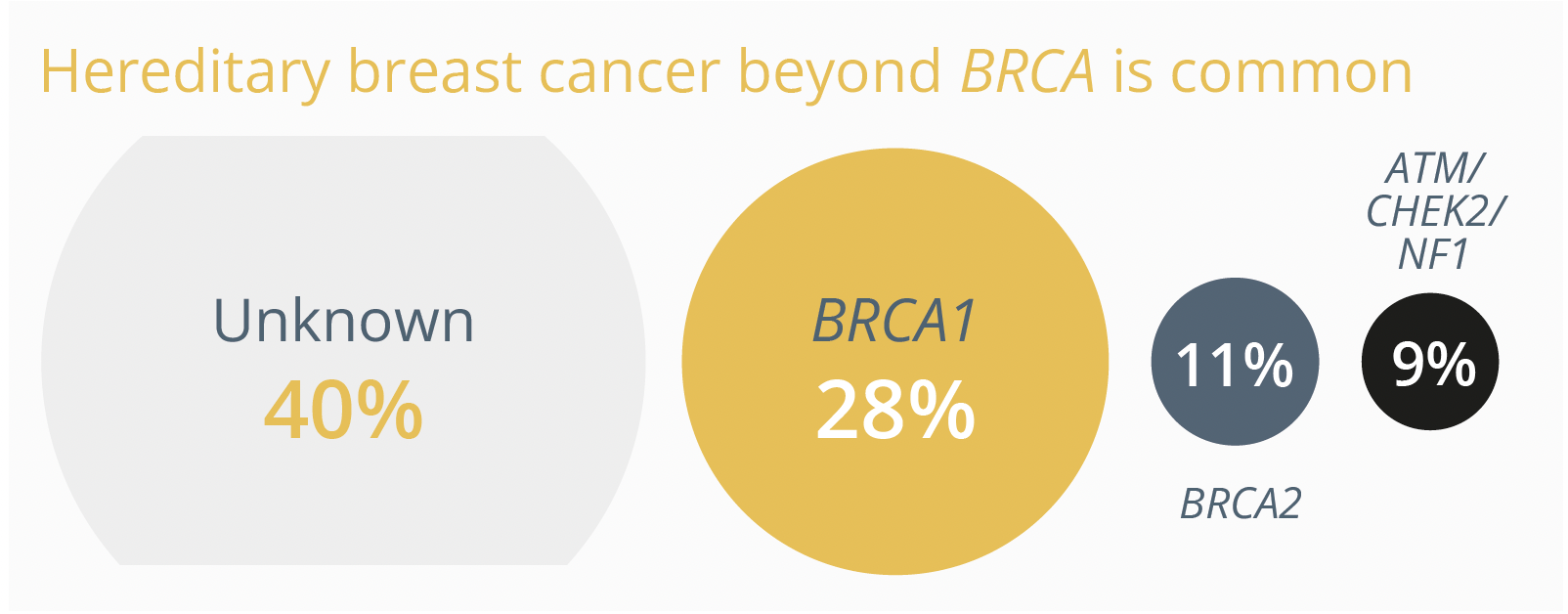
The percentage of different hereditary breast cancer genes associated with causing the disease.
“It is not only important for the patient who is in front of you, but you can also have important implications for relatives.”
- Kevin Punie, Leuven, Belgium
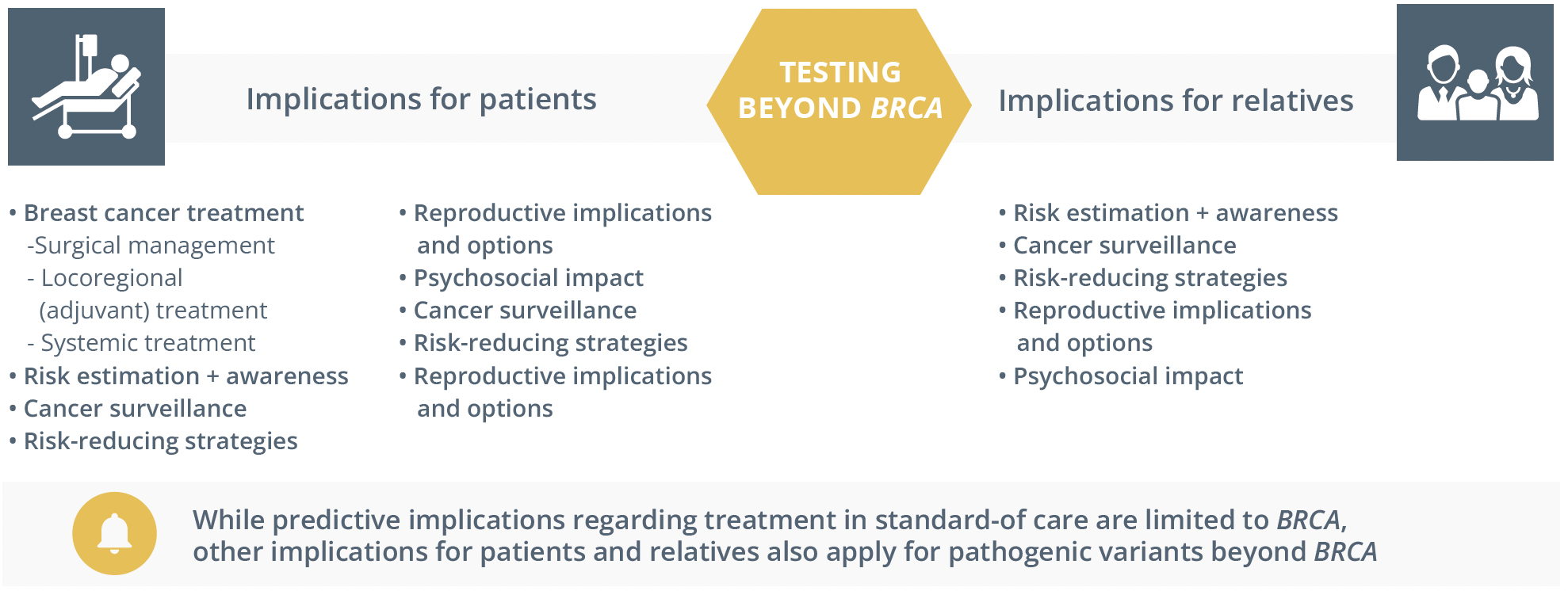
The implications of testing beyond BRCA for both patients and relatives
He added that for some genes beyond BRCA with high penetrance there can be reproductive implications and urged healthcare professionals to “think beyond the cancer implications”.
He moved on to present the clinical practice guidelines for breast cancer screening and prevention, specifying that there are no specific guidelines regarding which patients to test beyond BRCA. Risk-based testing criteria, for example, the national comprehensive cancer network (NCCN) has been developed for high penetrance breast cancer susceptibility genes such as BRCA1, BRCA2, CDH1, PALB2, PTEN and TP53. It is based on factors such as breast cancer history, familial antecedents of breast cancer, other personal or familial cancer antecedents and ancestry.
ESMO clinical practice guidelines for breast cancer prevention and screening
Risk-based testing criteria (e.g. NCCN): developed for high-penetrance breast cancer susceptibility genes (BRCA1, BRCA2, CDH1, PALB2, PTEN, TP53) and based on
- - Personal breast cancer history: age of onset, breast cancer subtype
- - Familial antecedents of breast cancer
- - Other personal or familial cancer antecedents
- - Ancestry
He warned, “An indication for genetic counselling is not an indication for genetic testing - you can only test a patient who agrees to test”. He explained that some syndrome-specific guidelines for testing exist and can be broader or narrower than breast cancer specific guidelines, so these breast cancer guidelines do not always capture everything, for example, that germline disease can cause TP53 variants.
Dr Punie revealed that the optimal panel does not exist, but depends on the local management implications. He underlined that when testing beyond BRCA there is also a need to counsel beyond BRCA, emphasising the importance of discussing treatment implications, risk reduction strategies, uncertainties in regards to risks, the limitation of prevention strategies and reproductive implications. He strongly stressed that multidisciplinarity is vital to find the optimal strategy and enable the management of patients with germline cancer predisposition.
Systemic treatment implications – the medical oncologist’s perspective
Dr Shani Paluch-Shimon, Jerusalem, Israel, drew attention to the challenge of identifying non- BRCA1/2 moderate-high penetrance genes, a phenomenon that is occurring more frequently. She emphasised that there are very limited data regarding these uncommon mutations from screening to disease course and responsiveness to treatment.
For choices of systemic therapy, Dr Paluch-Shimon presented the results from the TCBRC-048 phase II study (NCT03344965) showing that patients exclusively with germline mutations in PALB2 showed a high response rate and duration of response to olaparib. Beyond PALB2, however, she revealed that we don’t have much information about other pathogenic variants impact systemic therapy.
She warns, “as we use more multi-gene panels and we detect more pathogenic variants that are not BRCA, we need to remember that this is going to impact the whole spectrum of care".
She confirmed that germline testing has therapeutic implications in both early and advanced breast cancer, with emerging therapeutic implications beyond BRCA1/2. Currently Level 1 evidence is only for BRCA1/2 PARP inhibitors and good evidence for PALB2, but many trials are underway.

Clinical trials underway regarding therapeutic approaches for genes beyond BRCA.
Dr Paluch-Shimon ended her talk by reiterating the need to rethink our approach to genetic testing to ensure that it is timely and accessible as it may impact treatment decisions. Whilst further research is needed on the management and treatment of individuals with other moderate and high penetrance hereditary syndromes, she urged specialists to pool their knowledge together about these patients, their phenotypes, course of disease and response to treatments to be able to move forward.
“This will require an international effort to pool meaningful results from which we can draw conclusions.”
- Dr Shani Paluch-Shimon, Jerusalem, Israel
Surgical implications
Dr Peter Dubsky, Luzern, Switzerland, discussed the loco-regional treatment implications of genetic testing (BRCA and beyond) including use/non-use of radiotherapy in p53 carriers. In these patients, he explained, the use of irradiation can cause sarcomas and resistance due to gain of function mutations. He also advised against any ionising radiation (MRI over CT scans).
He then discussed the factors driving surgical decisions including age at diagnosis, current disease status, risk of recurrence, family history and co-morbidities.

The factors driving surgical decisions.
He then presented data about the risks that have been proven to predispose patients to recurrence. Whilst the data regarding BRCA1/2 clearly indicated lifetime risk up to 70% in certain timeframes, we now have data regarding other breast cancer risk genes also indicating cancer risk.
“There are two worlds here; there is a world that is BRCA1/2, TP53 and PALB2 which are odds ratios above 3. And then there is a world of moderate risk increases with very large confidence intervals.”
- Dr Peter Dubsky, Luzern, Switzerland
In response to these risk genes, Dr Dubsky answered a resounding yes to both early detection and lifestyle alterations, and confirmed a benefit of some medication but warned that surgery should be reserved for select patients.
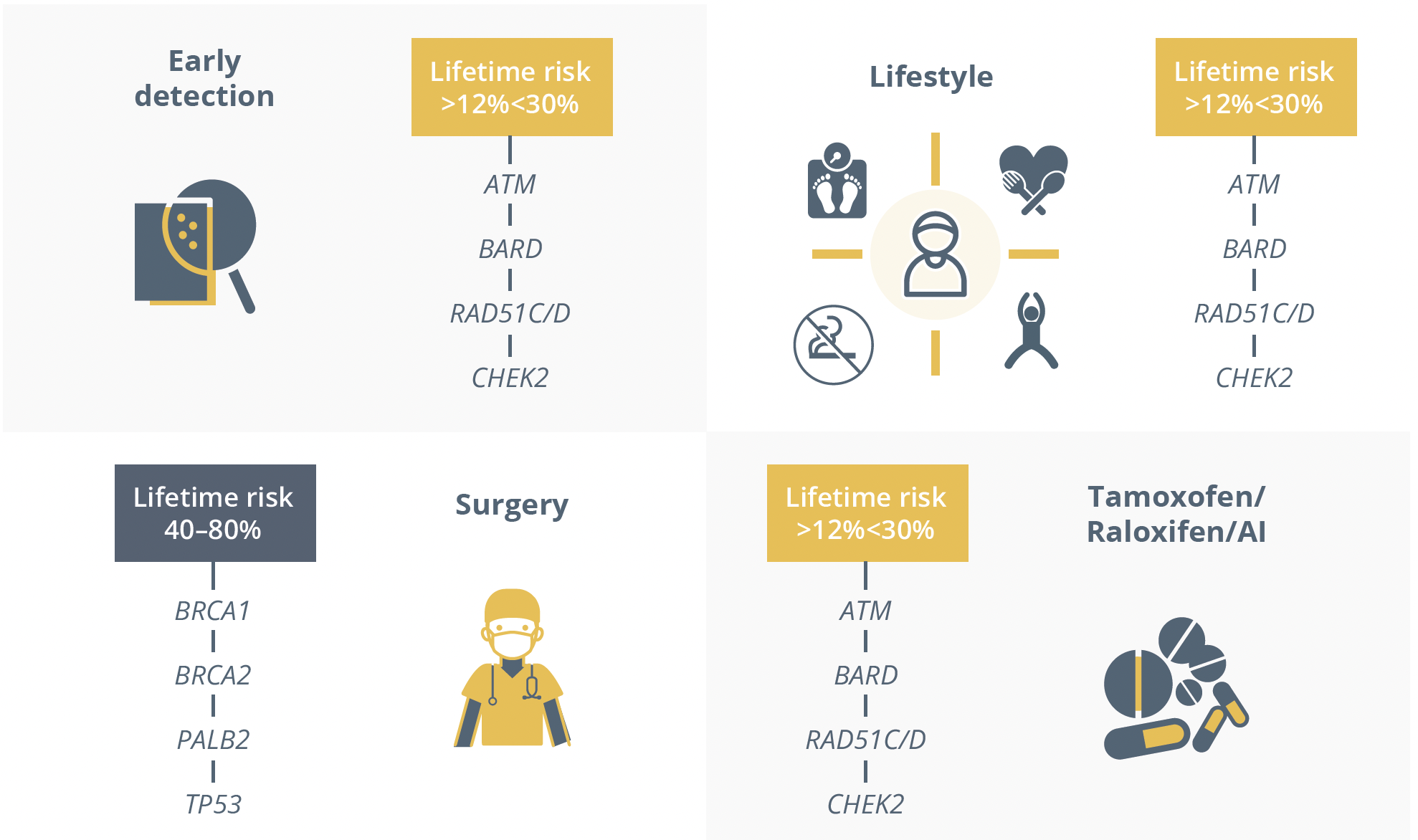
The 4 key aspects in preventing or managing breast cancer in patients with genetic predispositions.
He warned that moderate risk genes could be inhibited or changed by polygenic risk scores as well as family history, therefore treatment decisions based on their presence should be carefully considered.
“Do I think that not testing for moderate penetrance pathologic variants (PVs) is bad? No, I think sometimes it is perfectly justified.”
- Dr Peter Dubsky, Luzern, Switzerland
He explained that the lifetime risk of these PVs is uncertain, they have uncertain treatment implications, the ovarian risk elevation is negligible for most of these and the familial counselling is too complicated. He concluded his talk by praising the step-wise analysis used by Swiss practitioners, both feasible and adaptable to the clinical context of the patient and commended the complex work of the counsellors to translate this complex information to their patients.
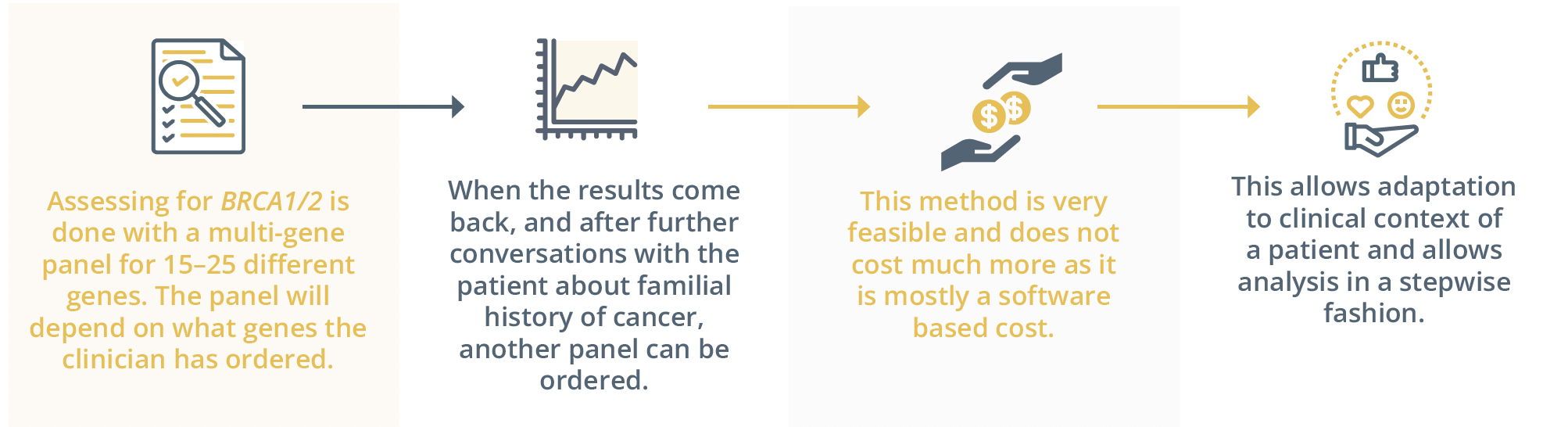
The step-wise analysis used by Swiss practitioners.
“As a counsellor, you have to keep in mind all of these incredibly complex scientific epidemiology data and you have to communicate them in a context that is very much mitigated by disease and risk and burden.”
- Dr Peter Dubsky, Luzern, Switzerland
New classification and staging of breast cancer
Introduction and scientific background
Dr Barbara Pistilli, Cedex, France, introduced the topic by presenting the results of the last decade, including the use of RNA-based multigene expression tests, the discovery of tumour infiltrating lymphocytes (TIL) as a prognostic biomarker and blood-based assays to detect minimal residual disease (MRD). She highlighted that the success of individualised therapy for patients with breast cancer really depends on the availability of new biomarkers.
- RNA-based multigene expression tests add to the standard immunohistochemistry and provide classifications into different molecular subtypes of prognostic and therapeutic significance.
- TILs represent a prognostic biomarker in early NBC and is now the first biological prognostic marker for early triple-negative breast cancer (TNBC) included in several international guidelines.
- Blood-based assay to detect MRD after curative treatments may allow to better identify patients at higher risk of recurrence and define early interventions aimed to prevent recurrences.
Rethinking tumour subtype classification: Learning from RNA expression
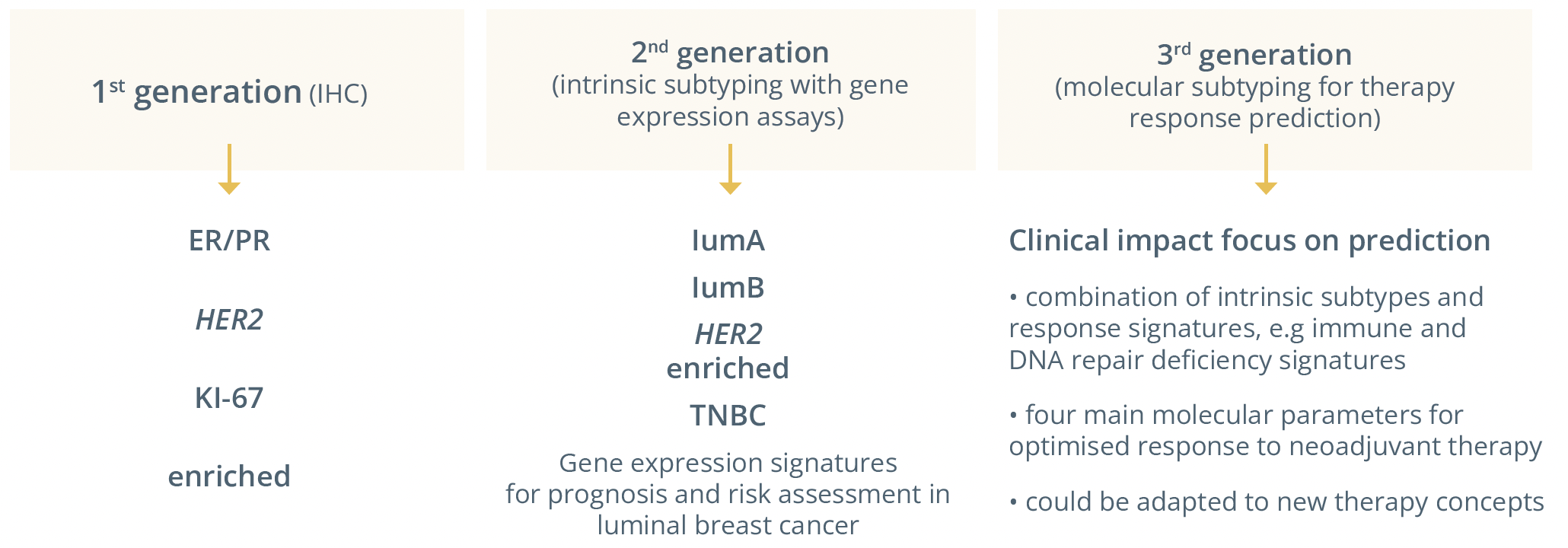
The 3 generations of tumour subtype classification methods.
Dr Carsten Denkert, Marburg, Germany, showed the methods for the classification of breast cancer subtypes, which led to the discovery of intrinsic subtypes and formed the basis for general therapy strategy. He added that RNA expression was useful to classify tumours based on complex RNA expression patterns with intrinsic subtypes forming the basis for current clinical breast cancer strategies.
“The (tumour) biology goes beyond what we see in immunohistochemistry.”
- Dr Carsten Denkert, Marburg, Germany
He presented the Penelope-B trial (NCT01864746), whereby patients (n=1250) underwent tumour subtyping before either receiving palbociclib or placebo. Whilst the prognostic subtyping worked well, there were changes in subtypes in pre- and post-therapy samples with 540 patients having paired pre- and post-neoadjuvant samples. In the group of patients with the same luminal B phenotype before and after therapy, palbociclib was shown to have an effect (not statistically significant). He warned that this phenomenon is not uncommon as biomarkers also change between primary and recurrent/metastatic tumours. Dr Denkert advised that future development needs to take into account molecular plasticity of tumour subtypes due to therapeutic pressure as well as the refined classification based on therapy response groups with the integration of single-cell information. Dr Denkert predicted what the 3rd generation of molecular subtyping would look like.
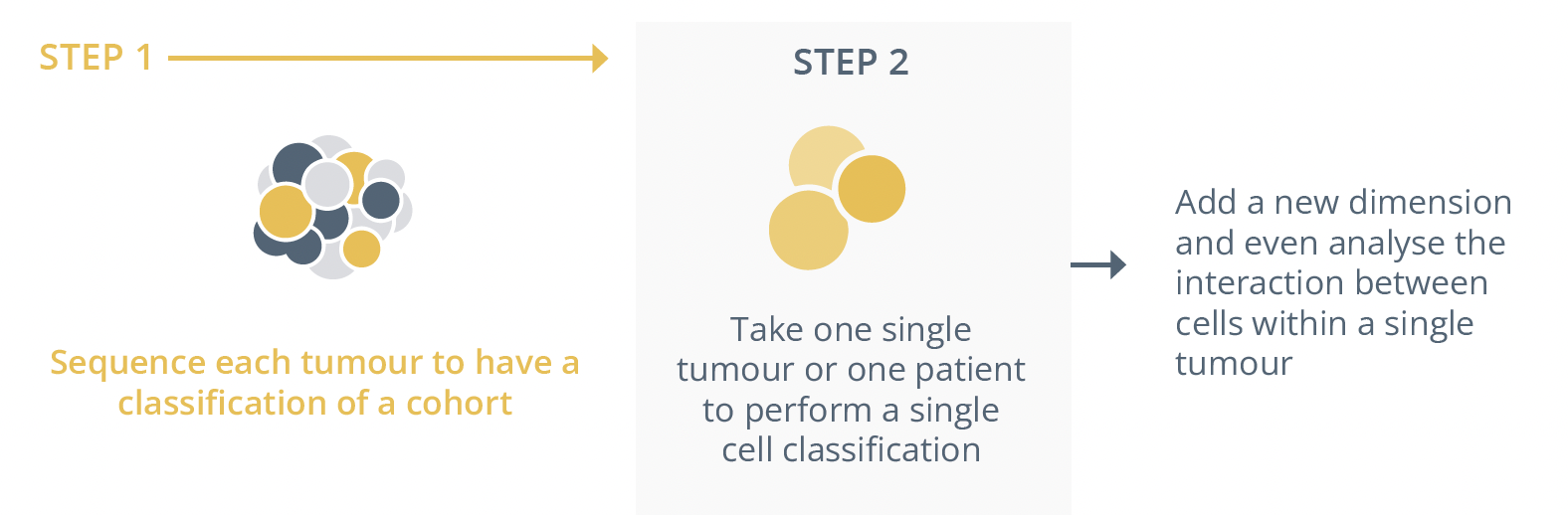
Steps for subtyping of malignant tumours with the addition of single cell sequencing
“For the 3rd generation it is very likely we will have a focus on clinical impact and on prediction that we will include additional therapies that are not included yet and have a revised classification starting with established markers but adding additional markers.”
- Dr Carsten Denkert, Marburg, Germany
Practically in the clinic he predicted there will be intrinsic subtyping as a basis but a gain of additional information from other techniques to add information regarding therapeutic decisions. These could include looking at immune gene activation, the DNA-damage response and next-generation sequencing (NGS) profiling.
“An adaptation of molecular subtyping may be done in the future to introduce response-predictive subtyping.”
- Dr Carsten Denkert, Marburg, Germany
Detecting MRD for new staging methods
Dr Nick Turner, London, United Kingdom, examined the use of blood-based assays to detect MRD, by sequencing the tumour to identify mutations subsequently tracked in plasma enabling the confident detection of any circulating tumour DNA (ctDNA) at low levels.
He suggested the potential prognostic value the ctDNA could add as it informs on grade and tumour size in patients with breast cancer. Furthermore, he explained that ctDNA detection post-neoadjuvant chemotherapy and surgery indicated a high risk of early relapse, but the sensitivity for detecting relapse was low. He explained this could be due to a low bulk of residual metastatic disease or a lack of ctDNA due to the post-chemotherapy tumour’s inability to release ctDNA until it starts to grow again.
“The main way to improve predictive capacity is to do molecular relapse detection, so serial monitoring for circulating tumour DNA in follow-up.”
- Dr Nick Turner, London, United Kingdom
Dr Turner explained that without sequencing of the tumour using these advanced genotyping assays could provide prognostic value but with lower discriminatory power, thereby limiting their use. He also warned against clonal haematopoiesis which could give false positives in ctDNA and revealed progress to generating tumour agnostic assays in the identification of tumour methylation patterns.
He presented a phase III trial in urothelial cancer, where ctDNA detection post-surgery enabled the detection of ctDNA positive patients that went on to benefit from atezolizumab – “doing liquid biopsies might enable you to identify a group of patients who benefit and can have their therapy escalated to try and prevent recurrence”. He equally presented evidence showing that this tool conversely could be used to de-escalate therapy.
Dr Turner concluded that while ctDNA-based monitoring can offer really highly confident predictions if a patient is going to relapse, there is currently no evidence yet of clinical utility or that treatment guided by ctDNA detection can improve outcomes. He reiterated that it was a clinical trial selection tool with multiple trials underway (i.e TRAK-ER) and planned but not at this time appropriate for routine clinical use.
The 3 Ws of early breast cancer management
Gene expression profiling and chemotherapy
In this educational session, Dr Aleix Prat, Barcelona, Spain, evaluated the current genomic tests for HR+/HER- disease. He confirmed they have high standards in terms of analytical validity as well as prognostic perspective in combination with other clinical pathological variables. However, these tests provided different levels of prognostic information and needed to be selected accordingly. In his opinion, Dr. Prat admitted that these tests could not predict chemotherapy benefit highlighted by the RxPONDER trial (NCT01272037), but could predict the absolute benefit of chemotherapy. The overall population had a statistically significant benefit of chemotherapy (p=0.026; adjusted HR: 0.81), however, from an absolute perspective this benefit was quite low. He moved on to talk about the effect of age/menopausal status on chemotherapy benefit highlighting trials TailorX (NCT00310180), MINDACT (NCT00433589) and RxPONDER, clarifying that this status does matter. He confirmed that more prospective data and further trials are needed.
“Gene expression by itself is not enough to predict prognosis.”
- Aleix Prat, Barcelona, Spain
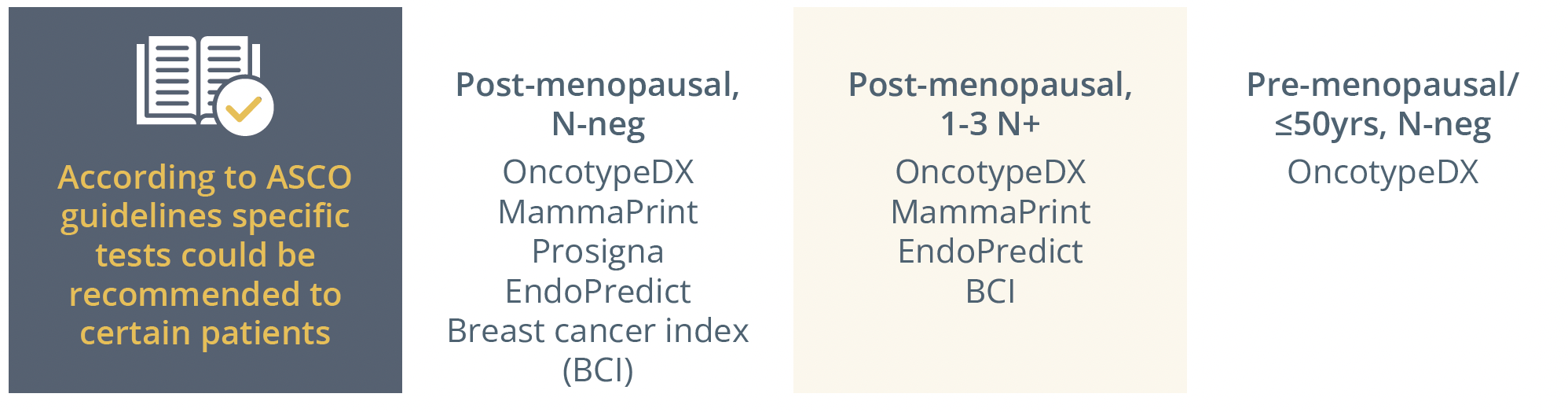
Genomic tests recommended for patients according to ASCO guidelines.
In clinical practice, if you have doubts about indicating chemo-based based on clinical-pathological data, a genomic test can help you make that decision:
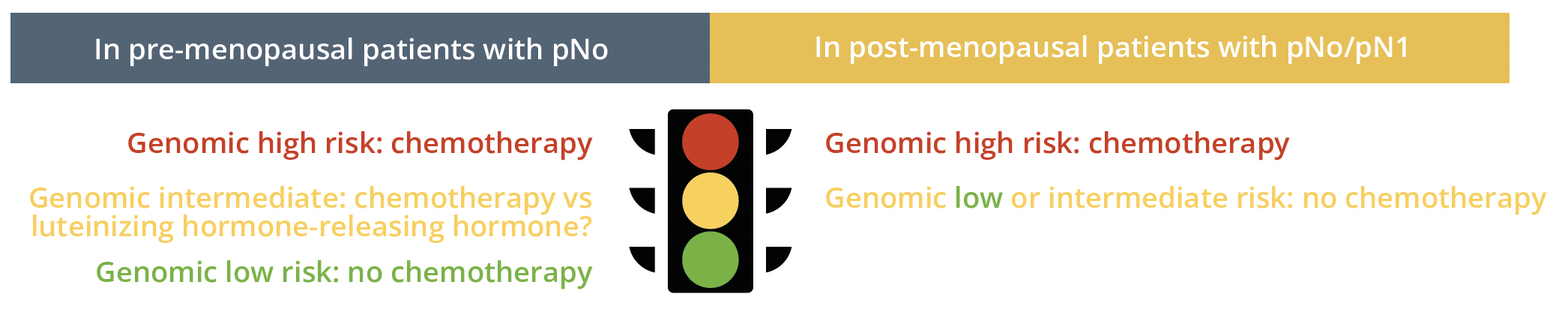
Genomic testing traffic light system for chemo indication in pre- and post-menopausal patients
He also addressed new approaches to identify outstanding outcomes such as testing the endocrine sensitivity of the tumour as this can provide value illustrated by the POIETIC study, defining which patients would or would not benefit from chemotherapy. Furthermore, pre-op endocrine therapy and molecular characterisation of residual disease after neoadjuvant endocrine therapy (ET) based therapies might help further identify the long-term risk of each patient.
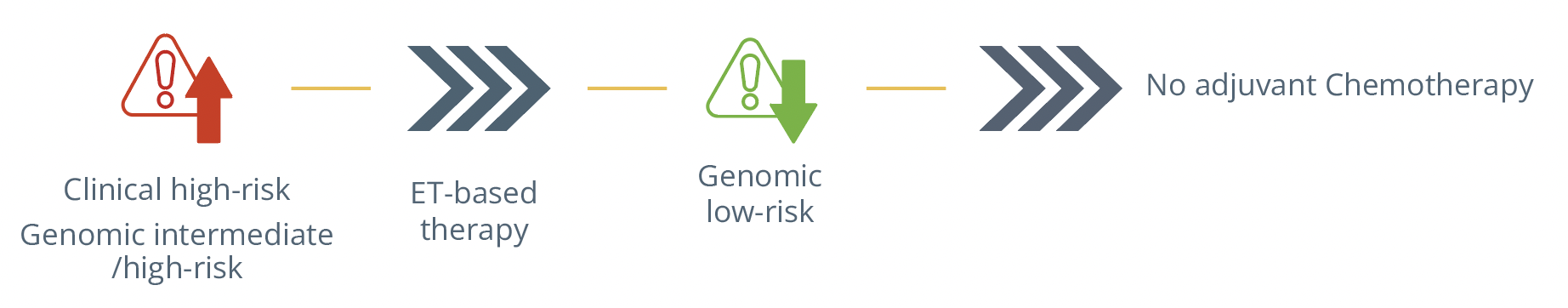
Therapeutic approach for patients depending on their clinical and genomic risk level.
Neoadjuvant therapy in luminal breast cancer: Who to treat, which regimen, and what duration?
Dr. Nadia Harbeck, Munich, Germany, advised against the use of neoadjuvant therapy in luminal breast cancer, as is stated in the ESMO guidelines. Neoadjuvant chemotherapy should be given to patients if there is a chemotherapy indication, which is decided based on tumour burden and biology. She underlined the use of gene expression analysis and endocrine response assessment to help guide decision-making – the latter being an inexpensive and informative test that can guide therapeutic choice. She added that gene expression assay from the core biopsy could equally be performed to aid in decision making (ADAPT study).
In regards to the chemotherapy used, she emphasised the use of the anthracycline-taxane sequence as it has been shown to have better effects than anthracycline alone. Anthracycline-free chemotherapy has been shown to be beneficial in cases of limited disease burden by the pooled analysis of studies PlanB and SUCCESS C. She praised the use of dose-dense chemotherapy and stressed the need to give the entire dose intensity before surgery. She explained how to calculate the residual risk after neoadjuvant chemotherapy as well as what to do if patients are classed as high risk post therapy with adjuvant abemaciclib and olaparib having shown benefit for these patients.
Moving on toward neoadjuvant endocrine therapy, Dr Harbeck admitted that data were limited but suggested aromatase inhibitor (AI) therapy for post-menopausal patients with an optimal duration of 6–12 months. Dr Harbeck praised the endocrine-based approaches with promising results and further studies (ADAPT) already ongoing but reiterated there is still lack of data to make this a clinical standard yet.
Dr Harbeck also discussed the potential benefits of short preoperative endocrine therapy during 3 weeks before surgery, with many small trials and data showing that it is clinically helpful (IMPACT, ACOSOG Z1031, and NEWEST).
She specified that pre-operative endocrine monotherapy is for restrictive cases such as elderly patients or those refusing chemotherapy adding that it is clinically helpful in both pre-menopausal(gonadotropin releasing hormone [GnRH] + AI/tamoxifen for 4 weeks) and post-menopausal (using AI) patients and can equally give information on the endocrine response.
Adjuvant endocrine therapy: who needs more than 5 years, which regimen, and what duration beyond 5 years?
Dr Evandro de Azambuja, Brussels, Belgium, began his talk by contextualising luminal cancer as it represents 70% of all breast cancer subtypes. He addressed the question of how to decrease the risk of relapse during the first 5 years and beyond highlighting the benefit of adding AIs to therapy as they could reduce recurrence rates as well as mortality. He mentioned other studies adopting different approaches to reduce relapse rates including:
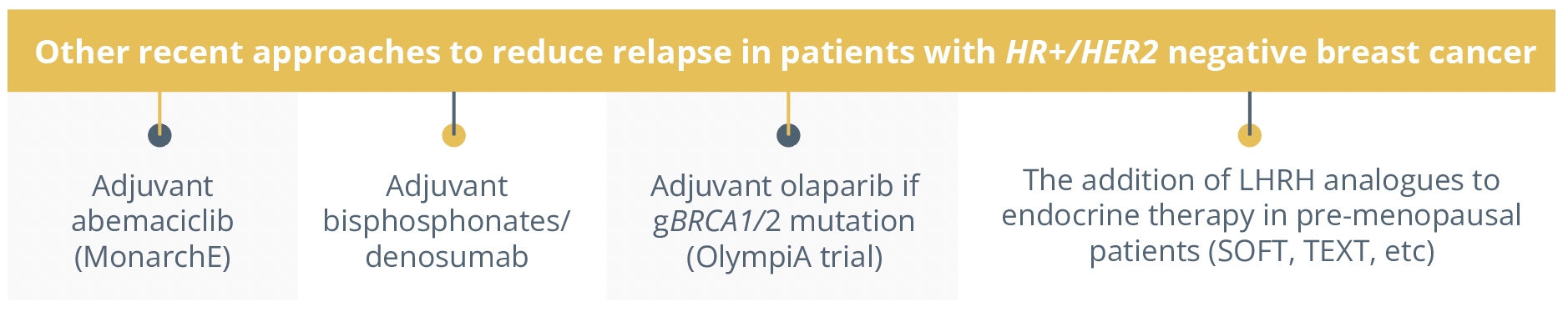
recent approaches to reduce relapse in patients with HER+/HER2 negative breast cancer.
Dr de Azambuja then answered the question on how to decrease the relapse risk during years 5 to 10, explaining that extended endocrine therapy beyond 5 years improved disease free survival (DFS) in patients pre-treated with tamoxifen (TAM) or AI as highlighted by trials GIM4 (NCT01064635), DATA (NCT00301457) and NSABP B-42 (NCT00382070).
The benefits of extending ET to 10 years showed no added benefit as shown by the IDEAL and ABCSG-16 trials (NCT00295620). He advised that the decision of extending ET should be based on relapse risk, tolerance, long-term side effects and patient preference. Dr de Azambuja also explained the tools to help predict late risk of relapse and benefit of extending ET including the Breast Cancer Index (BCI) and CTS5 calculator for post-menopausal patients.
According to the St. Gallen Consensus 2021, optimal duration of ET is 5 years for very low and low risk patients, 7–8 years for intermediate to high-risk patients and up to 10 years for very high risk patients. Dr de Azambuja stressed that AIs must be part of the treatment strategy in the post-menopausal patients except for very low risk patients or those with contraindications. He specified that patients who received 5 years of TAM with or without ovarian suppression (w/wt LHRH) should be considered for extended 5 years of TAM or AI depending on menopausal status, but ovarian suppression should not be extended beyond 5 years as there is limited data on this.
How to incorporate digital medicine in survivorship care after breast cancer
In this interactive Challenge your Expert session, Dr Ann Partridge, Boston, USA, gave us an overview on how to incorporate digital medicine in survivorship care. Dr Partridge began by highlighting that there is little patient-clinician contact in between follow-ups and routine tests and that digital medicine could help fill this void.
“Few oncology practices are equipped to anticipate, monitor or manage supportive care outside of these visits, especially in the survivorship period.”
- Ann Partridge, Boston, USA
She presented one study published in JAMA earlier this year, reporting benefits for metastatic patients using electronic symptom monitoring. She presented a complex mind map illustrating the multiple facets of survivorship.
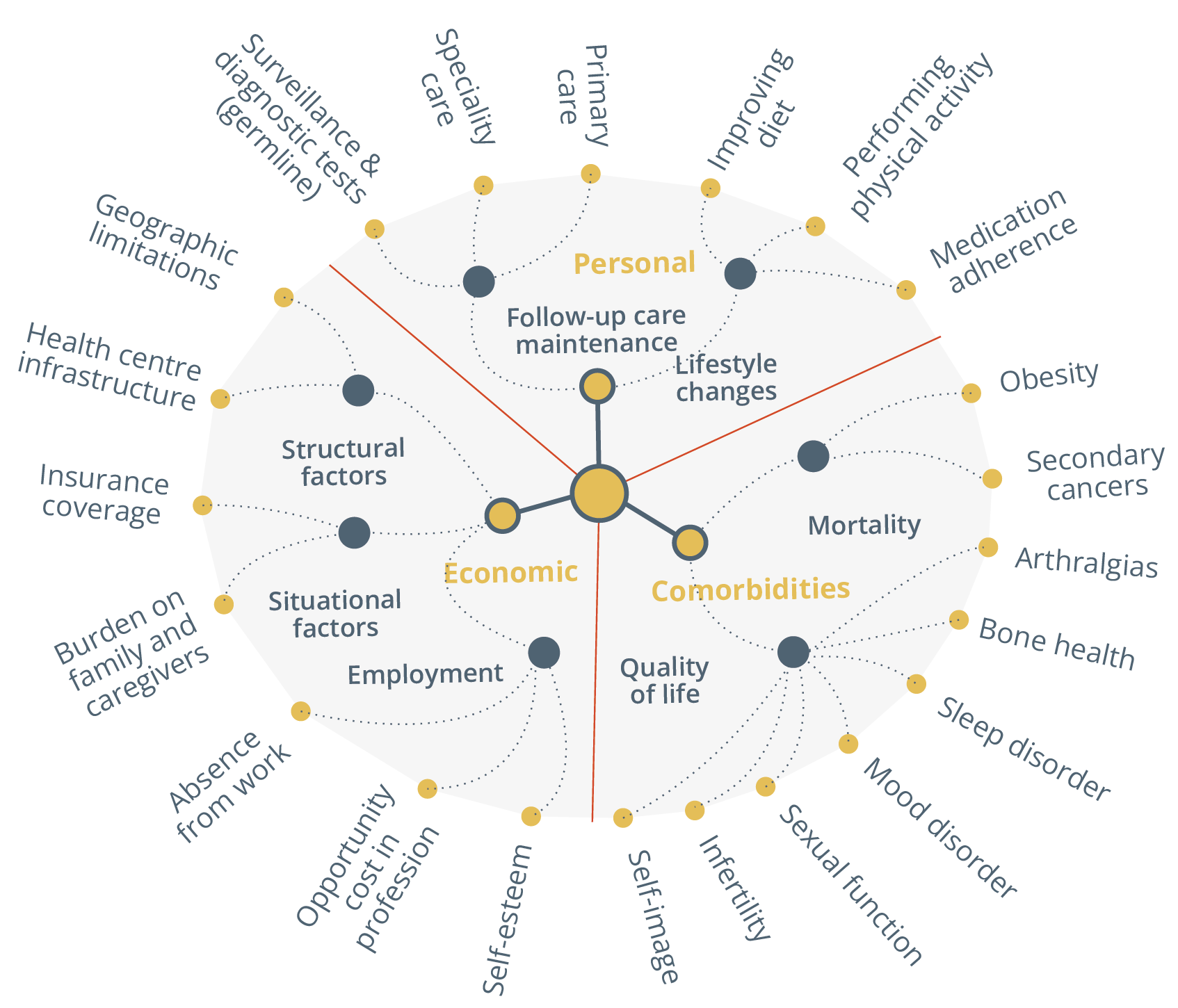
Mind map of survivorship
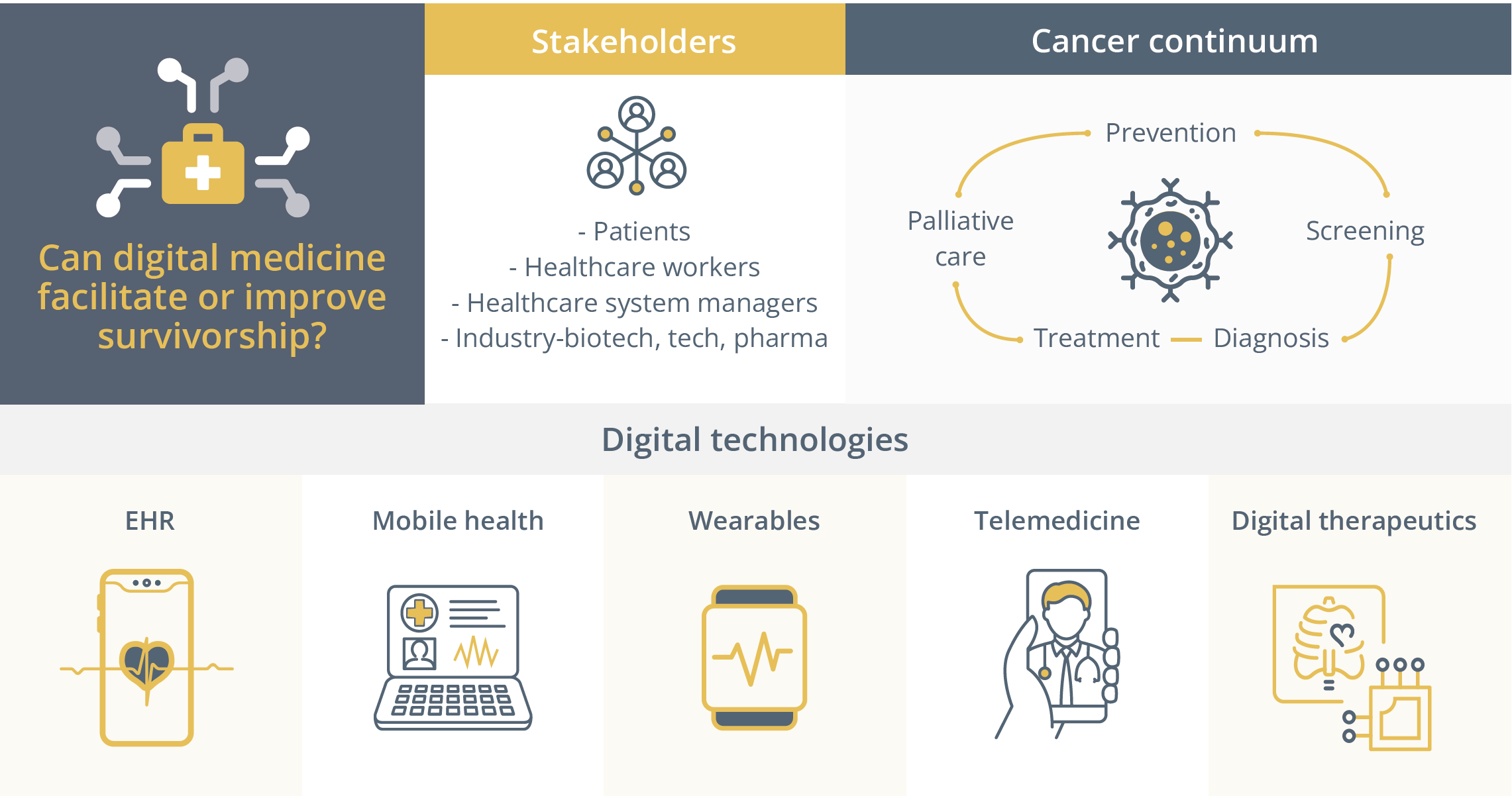
The use of digital medicine to facilitate or improve survivorship.
Data showed that digital monitoring could lead to improvements in breast cancer patients such as increased physical well-being, self-efficacy, quality of life and reduction in symptom burden. Self-management tools such as those that are internet-based and delivered remotely have also shown to improve some outcomes especially for individual symptoms and problems.
These outcomes included physical effects, psychosocial effects and health promotion/disease prevention. Whilst the data pointed to a benefit of such tools, Dr Partridge’s interaction with the audience highlighted that referral of patients to technology based portals is uncommon.
“The truth is we need to build systems that refer the patients.”
- Ann Partridge, Boston, USA
She excitedly presented ongoing efforts to incorporate these tools outside the clinic without heavy doctor or system involvement such as the ImPROVE program to improve the quality of care around the surgical experience so the surgical team understands pre-, peri- and post-op feedback from the patient. Dr Partridge also presented the Young, Empowered and Strong (YES) web-based portal helping young patients to self-monitor and self-manage concerns and symptoms through tailored information, resources and support. They can receive prompts, track their progress to monitor physical and emotional progress, engage in a chat room and produce creative/expressive writing in a journal.

The YES web-based portal tools.
Interestingly, in their YES pilot study, she revealed that the major concerns for patients were the so-called “soft” problems highlighting a gap in patient care. She praised the ability of these platforms to attain hard to reach populations such as busy adolescents, rural, underserved and more broad survivorship patients. She then discussed the next innovation technology in the pipeline, describing potential variables to measure, opportunities to obtain passive data and digital phenotyping. However, Dr Partridge warned that these interventions depended on patient engagement and adherence and that this interface may widen disparities due to the digital divide.
Congress highlights
Metastatic breast cancer
In the congress highlights, Dr Giuseppe Curigliano, Milan, Italy, discussed studies on metastatic breast cancer (MBC). The MONARCH 3 study (NCT02246621) assessed the efficacy of abemaciclib with anastrazole or letrozole versus placebo with anastrazole or letrozole in patients with endocrine-sensitive HR+/HER2- metastatic breast cancer.
The addition of abemaciclib significantly increased (p<0.0001) progression free survival (PFS) but overall survival data were not statistically significant. With a longer follow-up, however, he suggested that there could be a potential statistically significant and clinically meaningful benefit.
For endocrine-resistant HR+/HER2- MBC, the ELAINE 1 trial examined the potential drug repositioning of lasofoxifene versus fulvestrant. Whilst PFS and objective response rate were not statistically significant (p=0.138 and p=0.12, respectively), lasofoxifene had promising clinical activity and a good toxicity profile.
For HER2+ MBC, the PHILA trial (NCT03863223) assessed the efficacy of pyrotinib or placebo in combination with trastuzumab and docetaxel. Results showed a dramatic improvement in PFS (p<0.0001) but high levels of treatment related adverse effects (TRAE) such as diarrhea.
Finally, in triple-negative MBC, the UCBG3-06 START trial (NCT03383679) randomised patients to receive darolutamide or capecitabine. Whilst 46 patients (48%) discontinued darulotamide due to progressive disease, darulotamide monotherapy was associated with a clinical benefit rate (CBR) of 24.5% at 16 weeks, whereas capecitabine was associated with a CBR of 47.8%.
Closing Remarks
Following a successful in-person meeting this year with ~23,000 attendees, ESMO 2023 will continue to disseminate the latest cutting-edge data and provide a unique networking opportunity for oncology professionals in-person in Madrid, Spain, from 20–24 October 2023. Save the date for what will be an equally engaging and successful meeting in 2023.