
The Annual Meeting of the American Society of Clinical Oncology (ASCO), now in its 56th year, took place under a new virtual format on 29–31 May 2020. Opening the meeting, ASCO President Howard A. “Skip” Burris III highlighted that although COVID-19 had changed the format of this year’s ASCO 2020, the collective determination to make progress against cancer was as strong as ever.

ASCO President Howard A. “Skip” Burris III
Dr Burris explained that the COVID-19 pandemic has changed the way patients with cancer receive care and has encouraged healthcare professionals in the oncology community and their patients to weigh the risks and benefits of delaying treatment.
Dr Burris highlighted that data on how COVID-19 was impacting patients was being collected through the new ASCO Registry and CancerLinQ database, which this year crossed a milestone of 2.5 million patients.
As ever, this year’s ASCO meeting aimed to enable clinicians to understand how and when to integrate novel research into patient care. Dr Burris noted that the galvanising theme of the ASCO20 Virtual Scientific Program – “Unite and Conquer: Accelerating Progress Together” – would take on even more resonance given the extraordinary times as ASCO’s global audience learnt about new developments in cancer care.
“The world is grappling with a pandemic and we are all readjusting to a new reality, but it cannot stop us. We, the ASCO Community, are absolutely unwilling to let anything stop us in the fight against cancer.”
Howard A. “Skip” Burris III, ASCO President
Genitourinary Cancer
Urothelial Cancer
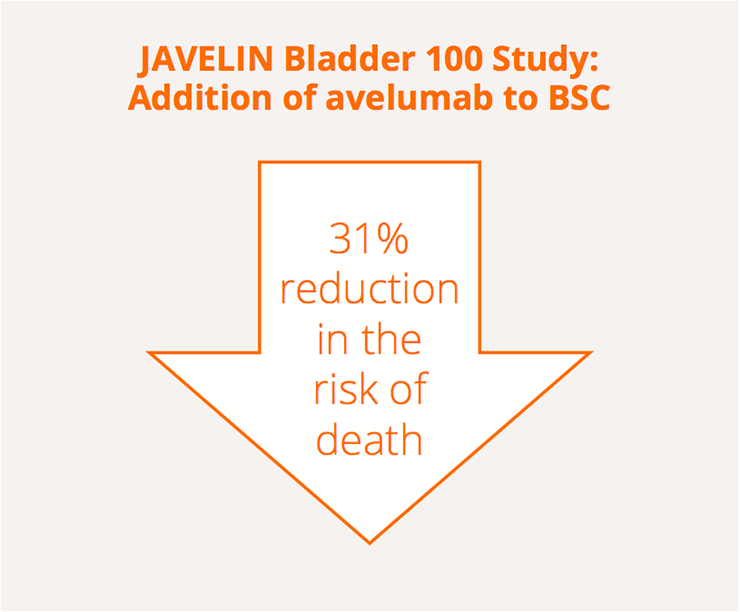
A reduction in the risk of death was observed in the JAVELIN Bladder 100 study
While platinum-based chemotherapy is an active first-line regimen for advanced urothelial carcinoma (UC), progression-free survival (PFS) and overall survival (OS) are often short due to chemotherapy resistance. Interim data from the Phase III JAVELIN Bladder 100 study, presented by Thomas Powles, Barts Cancer Institute, London, UK, demonstrated that maintenance therapy with the immune checkpoint inhibitor avelumab significantly improved survival outcomes compared with best supportive care (BSC) for patients with advanced UC who had not progressed after platinum-based induction chemotherapy (follow-up of 19.6 and 19.2 months, respectively) (LBA1; NCT02603432).
The study included patients with locally advanced unresectable or metastatic UC who had a complete or partial response, or stable disease, after standard first-line chemotherapy (gemcitabine and cisplatin or gemcitabine and carboplatin). Median OS durations were 21.4 and 14.3 months for the avelumab and BSC alone groups, respectively.
These findings were similar in those patients (51%) who were PD-L1-positive, with a 44% reduced risk of death favouring avelumab, although median OS has not yet been reached (versus 17.1 months for the BSC arm). The use of avelumab also significantly reduced PFS compared with BSC alone (hazard ratio [HR] 0.62 vs. 0.56, respectively). Median PFS durations in the total study population were 3.7 and 2.0 months in those patients who received avelumab with BSC or BSC alone, respectively. The safety profile of avelumab with BSC was reported to be manageable and consistent with previous monotherapy studies.
“This is the first frontline trial in UC to show a significant survival signal.”
Thomas Powles, Barts Cancer Institute, London, UK
Elizabeth R. Plimack, Fox Chase Cancer Center, Philadelphia, USA, highlighted that this was the longest OS ever documented in a Phase III metastatic UC trial, in any line of therapy. However, Dr Plimack noted that it was unlikely that a non-responder to platinum would have benefitted from frontline checkpoint inhibition, even if programmed cell death ligand 1 (PD-L1)-positive.
Data from the Phase III IMvigor010 study failed to show any significant improvement in disease-free survival (DFS) with the use of adjuvant atezolizumab in patients with high-risk muscle-invasive UC according to Maha Hussain, Robert H. Lurie Comprehensive Cancer Center, Chicago, USA (Abstract 5000; NCT02450331). Patients had previously undergone radical cystectomy or nephroureterectomy with lymph node dissection. In this primary analysis, patients who received adjuvant atezolizumab achieved a DFS of 19.4 months which was comparable with 16.6 months for those who received observation alone. OS follow-up is currently ongoing.
No significant differences in DFS were reported when patients were subgrouped by:
- PD-L1
- Disease site/stage
- Pathologic node status
- Prior use of neoadjuvant chemotherapy
“IMvigor010 is the first Phase III study to evaluate the benefit of an adjuvant checkpoint inhibitor in muscle-invasive UC.”
Maha Hussain, Robert H. Lurie Comprehensive Cancer Center, Chicago, USA
The combination of pembrolizumab and axitinib has continued to demonstrate superior and durable antitumour activity compared with sunitinib in treatment-naïve patients with first-line advanced renal cell carcinoma (RCC), according to an updated analysis of the Phase III KEYNOTE-426 study (Abstract 5001; NCT02853331). After a median follow-up of 27 months, 24-month OS was significantly greater with pembrolizumab and axitinib compared with sunitinib (74% vs. 66%, respectively). Median OS has not yet been reached with pembrolizumab and axitinib compared with 35.7 months for sunitinib. PFS was also significantly improved with pembrolizumab and axitinib versus sunitinib (15.4% vs. 11.1%, respectively). The clinical benefit of pembrolizumab and axitinib was observed in all subgroups tested, including International Metastatic RCC Database Consortium risk and PD-L1 expression subgroups.

24-month OS in patients with advanced RCC receiving pembrolizumab + axitinib versus sunitinib
“An exploratory landmark analysis demonstrated that greater depth of tumour shrinkage was associated with increased OS in the pembrolizumab and axitinib arm.”
Elizabeth R. Plimack, Fox Chase Cancer Center, Philadelphia, USA
Closing Remarks
In response to the COVID-19 pandemic, ASCO completely reimagined and reinvented its 2020 Annual Meeting by delivering a successful virtual meeting. This virtual platform still had the aim of achieving an engaging and educational meeting experience for participants from around the world. Delegates were able to view data, clinical experiences, patient perspectives and best practices that will hopefully stimulate new ways of thinking and ultimately translate into optimal patient care within the oncology field.
©Springer Healthcare 2020. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.