Types of Water for the Reconstitution of CellCept Oral Suspension
This letter responds to your request for information on CellCept® (mycophenolate mofetil) oral suspension and the types of water that can be used for reconstitution.
Refer to the preparation instructions provided in the locally approved product label for more information. Any deviation from this information is considered off-label and any treatment decisions based on such deviations are the full responsibility of the prescribing physician.
Last updated February 03, 2025
Recommended types of water to use for reconstitution
Table 1. Types of water and whether they are appropriate for reconstituting CellCept oral suspension
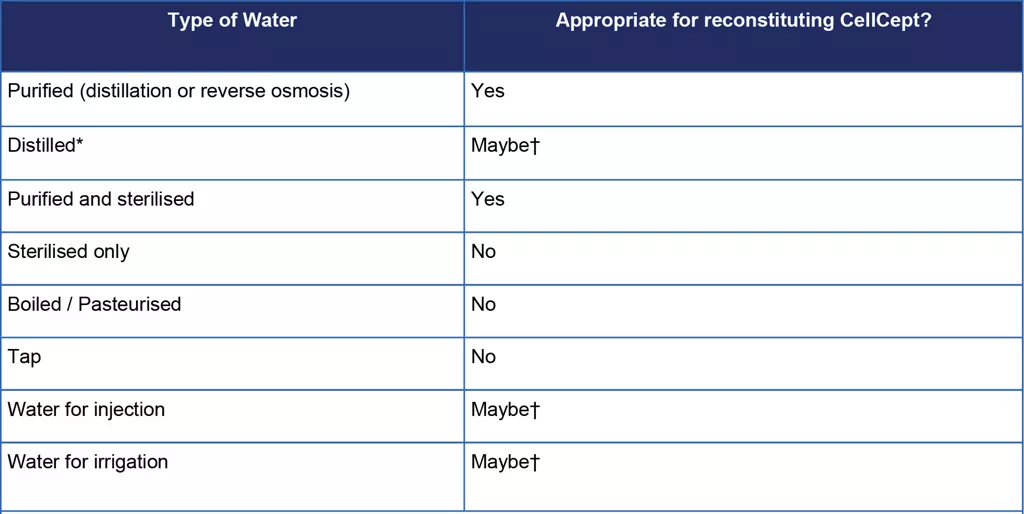
Notes: *Distilled water is water purified by distillation. †Distilled water, water for injection, or water for irrigation may be appropriate if it is purified water. The pharmacist or Healthcare Professional (HCP) would need to double check with the supplier.
Roche has physical and chemical stability data available for reconstitution of CellCept oral suspension with purified water only and cannot recommend reconstitution with any other type of water.
US Pharmacopeia (USP) defines purifed water as meeting the requirements for ionic and organic chemical purity and must be protected from microbial contamination.[1] General Chapter <1231> of the USP, Water for Pharmaceutical Purposes, states that "the minimal quality of source water for the production of Purified Water is drinking water whose attributes are prescribed by the US EPA, EU, Japan, or WHO. This source water may be purified using unit operations that include deionization, distillation, ion exchange, reverse osmosis, filtration, or other suitable purification procedures."
Any water which meets the requirements for purified water as prescribed by the US EPA, EU, Japan, or WHO should be ok to use in the preparation of CellCept Syrup.
Importance of water purity
The purity of the water used to reconstitute CellCept oral suspension is an important consideration.[2] Many patients treated with immunosuppressant drugs (including CellCept) are at increased risk of opportunistic infection (bacterial, fungal, viral, and protozoal).[3] Therefore, only purified water should be used to reconstitute CellCept.
References
- United States Pharmacopeia (USP) <Chapter 1231> Water for Pharmaceutical Purposes. Available at http://www.uspbpep.com/usp29/v29240/usp29nf24s0_c1231.html. Accessed on April 30, 2024.
- Roche Internal Regulatory Document (Accessed on 26 Jun 2023).
- Roche Internal Communication (Accessed on 26 Jun 2023).
Medinfo
Need to contact Roche?
Request Product Information
Request Product Information
Ask us a question and request information about Roche products or services.
Report a potential side effect
Report a potential side effect
If you have experienced potential side effects with a Roche product you can report it here.
Report a potential product defect
Report a potential product defect
If you suspect a potential defect or a Roche product has not met your expectations you can report it here.
Request temperature stability assessment
Request temperature stability assessment
Request an assessment if your product was stored outside the recommended temperature range.
