Incidence and Management of Herceptin IV and Herceptin SC Administration Related Reactions
This article responds to your request for information on the incidence and management of administration-related reactions associated with Herceptin® (trastuzumab) and Herceptin SC ® (trastuzumab and hyaluronidase). This response was developed according to the principles of evidence-based medicine and contains data from clinical studies analysed in an internal safety report.
Last updated March 27, 2025
Summary
- ARRs have been observed following Herceptin IV and Herceptin SC administration. The rate of ARRs has varied between clinical studies depending on the indication, whether Herceptin was given concurrently with chemotherapy or as monotherapy, and data collection methodology.
- Herceptin SC has demonstrated an overall similar safety profile, including the rates of ARRs, to the known safety profile of Herceptin IV.
- Management of a patient who experiences an ARR can include
- stopping or slowing the Herceptin IV infusion
- provide supportive care in line with local practice, and
- pre-medicating subsequent doses of Herceptin with antihistamines, corticosteroids, or both.
- Literature on the management of ARRs associated with subcutaneous administration of biologics has been published.
Abbreviations
ARRs=administration-related reaction
EBC=early breast cancer
HER2=human epidermal growth factor receptor 2
ISR=injection site reaction
MBC=metastatic breast cancer
ARRs associated with Herceptin IV and Herceptin SC
The definition of ARRs has varied across Herceptin IV and Herceptin SC clinical trials, but includes[1]
- systemic infusion-related reactions
- systemic reactions associated with SC administration, and
- injection site reactions or hypersensitivity.
Systemic reactions have been seen in all Herceptin IV and Herceptin SC clinical trials, including[2]
- chills and/or fever
- dyspnoea
- hypotension
- wheezing
- bronchospasm
- tachycardia
- reduced oxygen saturation, and
- respiratory distress.
Local reactions are symptoms experienced at the site of the injection, such as
- erythema
- pruritus
- oedema
- rash, and
- pain.[3]
Incidence of ARRs associated with Herceptin IV and Herceptin SC in clinical trials
The rate of ARRs of all grades has varied between studies depending on
- the indication
- whether Herceptin was given concurrently with chemotherapy or as monotherapy, and
- data collection methodology.[2]
Anaphylactoid reactions have been observed in isolated cases.
An analysis of the data from 8 individual clinical trials and a pooled analysis reviewed the frequency and severity of ARR events in patients who have been treated with Herceptin IV or Herceptin SC in clinical trials.[4] The analysis reviewed the rates and severity of ARRs in EBC and MBC patient populations.
ARRs in MBC patients treated with Herceptin in clinical studies
Data was retrieved from an internal analysis of 9 Herceptin IV clinical studies in MBC patients, which included a pooled analysis of 6 studies and 3 separate studies.[4] The study designs included
- single-arm trials
- two-arm trials
- Herceptin used as a single agent, and
- Herceptin given in combination with chemotherapy.
The rates and severities of ARRs in MBC patients are summarised in Table 2.
Table 1. Rates of ARRs across Herceptin IV clinical studies in patients with MBC[4]
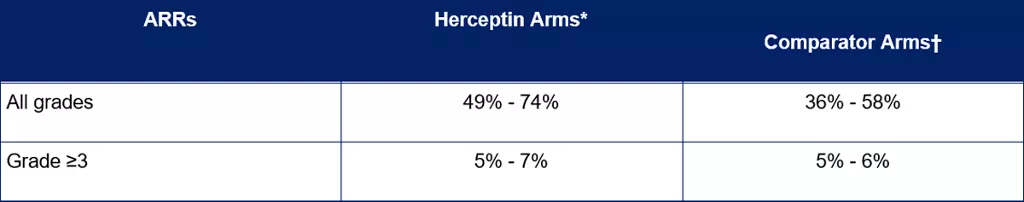
*Herceptin was given as a single agent or with chemotherapy. † Comparator arm included chemotherapy.
ARRs in EBC patients treated with Herceptin in clinical studies
Five Herceptin IV and Herceptin SC studies were included in the analysis on EBC patients.[4] All of the studies had a comparator or observation arm and the study designs included
- Herceptin in the adjuvant setting
- Herceptin in the neoadjuvant setting
- Herceptin used as a single agent, and
- Herceptin given in combination with chemotherapy.
The rates and severities of ARRs in EBC patients are summarised in Table 2.
Table 2. Rates of ARRs across Herceptin clinical studies in patients with EBC[4]
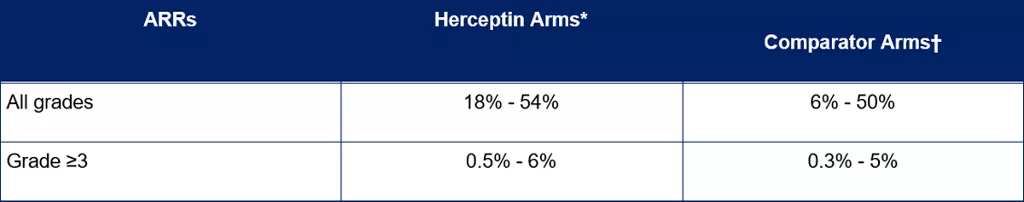
*Herceptin was given as a single agent or with chemotherapy. † Comparator arm included chemotherapy.
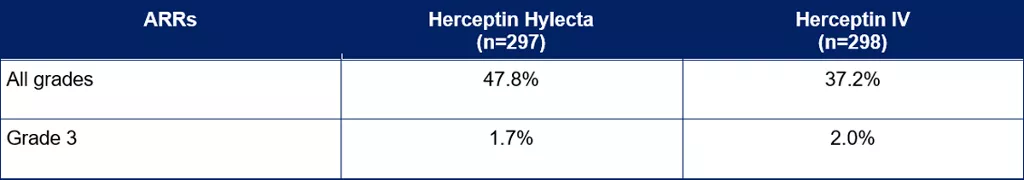
Rates of ARRs for Herceptin SC compared to Herceptin IV
The HannaH study was a Phase 3, non-inferiority, randomized, open-label study that compared the pharmacokinetic, efficacy, and safety of a fixed dose of Herceptin SC to Herceptin IV in the neoadjuvant and adjuvant setting in women with HER2-positive early breast cancer.[5] The HannaH study was one of the clinical trials included in the safety analysis above.[4]
The safety profile of Herceptin SC, including the rates of ARRs, was overall similar to the known safety profile of Herceptin IV.[2,5] The incidence of all grade and Grade 3 ARRs are presented in Table 2. No severe Grade 4 or 5 reactions were observed.
The systemic reactions included hypersensitivity, hypotension, tachycardia, cough, and dyspnoea.[3] The local reactions included erythema, pruritus, oedema, rash, and pain at the site of the injection.
Table 2. Comparison of ARRs between Herceptin SC and Herceptin IV[2]
Recommendations on the management of ARRs
If a patient experiences an ARR, symptoms can be managed in several ways:[1,2]
- Stopping or slowing the Herceptin IV infusion
- Provide supportive care in line with local practice. This could include, but is not limited to
- analgesic or antipyretic such as meperidine or paracetamol
- antihistamines such as diphenhydramine
- oxygen
- beta-agonists, or
- corticosteroids.
- Pre-medicate subsequent doses of Herceptin with antihistamines, corticosteroids, or both.
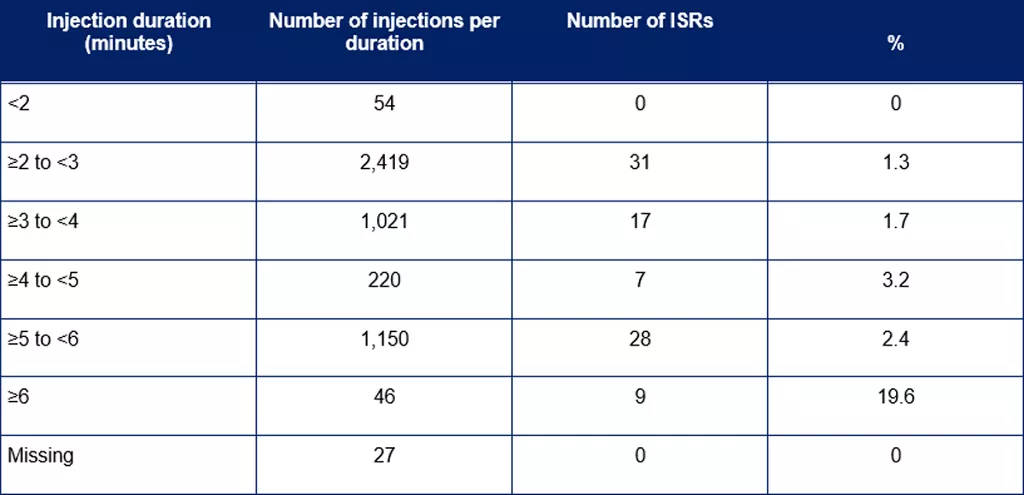
Correlation of ISRs with Herceptin SC injection time
A subanalysis of the HannaH study evaluated the incidences of ISRs and their correlation with injection duration of Herceptin SC.[3] The results, presented in Table 3, revealed that the proportion of ISRs was highest when injection time was ≥6 minutes.
Table 3. Correlation of ISRs with injection duration[3]
Published literature on the management of ARRs associated with SC administration of biologics
A search of the published literature identified several articles that discuss strategies for the prevention and management of ARRs associated with SC administration of biologic medicines.[6-8] Strategies include patient education, pain managment recommendations, a review of injection technique, and appropriate training. These articles are not specific to Herceptin SC and any recommendations are not endorsed by Roche or Genentech. Please note, the search was not exhaustive and other articles may have been published on this topic.
The interested reader is directed to the relevant references for the full articles.
References
- Roche Internal Clinical Report (IB 23.0). Accessed 4 October 2023.
- Roche Internal Regulatory Report (Herceptin CDS 21.0). Accessed 2 October 2023.
- Roche Internal Clinical Report (HannaH CSR). Accessed 2 Oct 2023.
- Roche Internal Safety Report. Accessed 4 October 2023.
- Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol 2012;13:869-78. https://www.ncbi.nlm.nih.gov/pubmed/22884505
- Eisenberg S. Subcutaneous Administration: Evolution, Challenges, and the Role of Hyaluronidase. Clin J Oncol Nurs 2021;25:663-671. https://www.ncbi.nlm.nih.gov/pubmed/34800095
- St CA, Prignano F, Goncalves J, et al. Understanding and Minimising Injection-Site Pain Following Subcutaneous Administration of Biologics: A Narrative Review. Rheumatol Ther 2020;7:741-757. https://www.ncbi.nlm.nih.gov/pubmed/33206343
- Fernandez J, Madsen S, Krase J, et al. Classification and mitigation of negative injection experiences with biologic medications. Dermatol Ther 2020;33:e13240. https://www.ncbi.nlm.nih.gov/pubmed/32012405
Medinfo
Need to contact Roche?
Request Product Information
Request Product Information
Ask us a question and request information about Roche products or services.
Report a potential side effect
Report a potential side effect
If you have experienced potential side effects with a Roche product you can report it here.
Report a potential product defect
Report a potential product defect
If you suspect a potential defect or a Roche product has not met your expectations you can report it here.
Request temperature stability assessment
Request temperature stability assessment
Request an assessment if your product was stored outside the recommended temperature range.
