Ocrevus and Pregnancy and Lactation in Women with Multiple Sclerosis
This article responds to your request for information on Ocrevus® (ocrelizumab) and pregnancy and lactation in women with multiple sclerosis.
Last updated March 20, 2025
Summary
- As of March 2024, 3,989 pregnancies had been reported in women with multiple sclerosis treated with Ocrevus.
- Available data do not suggest an increased risk of adverse pregnancy or infant outcomes with Ocrevus use.
- The Phase 4 MINORE study assessed maternal, fetal, and infant outcomes in women with multiple sclerosis who have been exposed to Ocrevus during the 6 months prior to their last menstrual period or at any time during their pregnancy.
- Exposure to Ocrevus during pregnancy did not result in infant B-cell depletion.
- There was minimal placental transfer and low exposure of infants to Ocrevus in utero.
- The SOPRANINO study is evaluating the pharmacokinetics of Ocrevus in the breast milk of lactating women with multiple sclerosis and the resultant exposure and outcomes in infants.
- The study met its co-primary endpoints, showing negligible Ocrevus transfer to infants in breast milk and no depletion of their B-cells.
Abbreviations
ADID=average daily infant dose
AE=adverse event
ARR=annualised relapse rate
CIS=clinically isolated syndrome
DMT=disease modifying therapy
GA=gestational age
GWk=gestational weeks
LLN=lower limit of normal
LMP=last menstrual period
MCA=major congenital anomaly
MS=multiple sclerosis
(P)PMS=(primary) progressive multiple sclerosis
RID=relative infant dose
RRMS=relapsing-remitting multiple sclerosis
Maternal-exposure pregnancies in patients treated with Ocrevus
Definition of in utero and maternal Ocrevus exposure
In utero exposure:
An embryo or fetus was considered to have been exposed to Ocrevus in utero if the last infusion occurred
- within the previous 3 months of conception,
- during pregnancy, or
- if the date of infusion was unknown.[1]
Maternal exposure:
Defined as having 1 or more Ocrevus infusions at any time point before conception or during the pregnancy.[1]
Roche global safety database
Dobson et al. described pregnancy outcomes of women who were exposed to Ocrevus in clinical trials in multiple sclerois (MS) and postmarketing experience up to March 28, 2024.[2] As of March 2024, more than 350,000 patients have been treated with Ocrevus globally.[3]
There were 3,989 maternal-exposure pregnancies in patients treated with Ocrevus for MS as of March 2024.[2] Of these pregnancies, 3,022 were prospective in nature (i.e., final outcomes were unknown at initial notification). Of the 1,502 cases with known outcomes, 1,268 (84.4%) resulted in live births: [2]
- 61.4% of pregnancies were full term
- 8.0% were pre-term
- 30.7% had an unknown gestational week (GWk)
Details of the outcomes among prospective cases are shown in Table 1. Across exposure categories, data were in line with expected epidemiological ranges.[4,5]
Table 1. Summary of pregnancies with known outcomes [2]
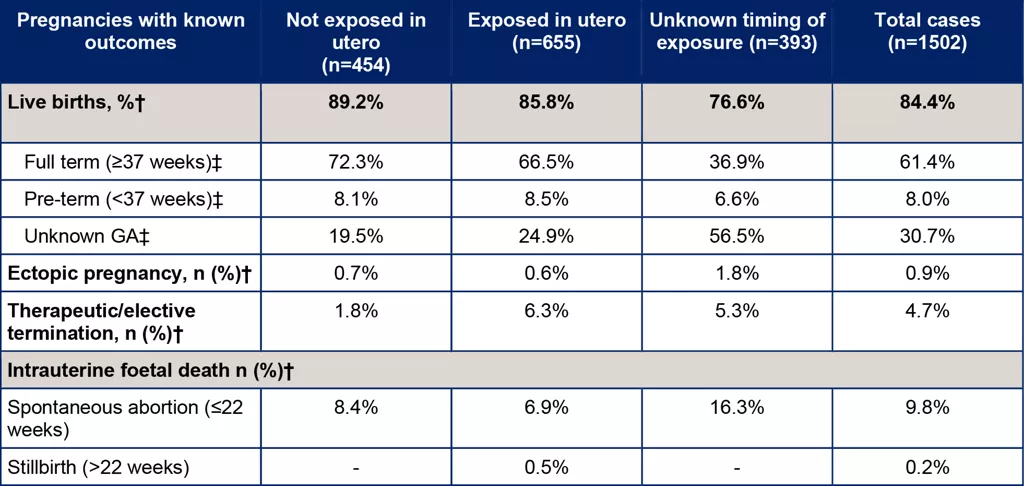
†Percentages represent fractions of the total known outcomes of the respective exposure category (not exposed in utero, exposed in utero unknown exposure, total). ‡Percentages represent fractions of the total live births for the respective exposure category (not exposed in utero, exposed in utero unknown exposure, total). The dash indicated that no cases were reported.
Major congenitial anomalies (MCAs) in pregnancies with known outcomes
There were 18 prospectively reported live and stillbirths with at least one major congenital anomaly (1.4%; 17 live births and 1 stillbirth); 2 live births reported 2 major congenital anomalies each, totaling 20 MCAs.
The most frequently observed major congenital anomalies were: urinary anomalies (6 cases) and congenital heart defects (5 cases).[2]
In the MS and general population, MCAs have been reported in 2.2-4.2% and 2.0-4.4% of children born each year, respectively.[4-8]
Integrated analysis from 13 interventional clinical trials
Vukusic et al. analyzed peripartum disease activity data from 13 interventional clinical trials of Ocrevus in 103 women with MS (100 RMS, 3 PMS) who delivered a live birth (CCOD November 24, 2023).[9] Women were required to hold off on Ocrevus treatment during pregnancy and breastfeeding (per trial protocol). The annualised relapse rate (ARR) remained low from 1 year prior to pregnancy through 1 year postpartum.
- The rate of clinical relapse (per Patient Years [95% CI]) was 0.06 (0.02–0.14) pre-pregnancy, 0.03 (0.00–0.09) during pregnancy and 0.04 (0.01–0.14) postpartum.
- Relapses were reported in 6 women pre-pregnancy, 2 women during pregnancy, and 2 women postpartum; the median time from Ocrevus treatment start to relapse was 23 months (range 7–28).
Phase IV MINORE study
Study design
MINORE is a Phase 4, prospective, multicenter, open-label study in women with CIS or MS, evaluating the placental transfer of Ocrevus, and the corresponding pharmacodynamic effects in infants.[10,11] The study enrolled 35 women between GWk 22 to 26, whose last Ocrevus dose occurred at any time from 6 months before the last menstrual period (LMP) until the end of the first trimester.
The primary endpoint of the study is the proportion of infants with B-cell levels below lower limit of normal (LLN) at Week 6 of life.
Key secondary endpoints are:
- serum Ocrevus levels in umbilical cord blood, and
- infant humoral immune responses to vaccinations.
Hellwig et al. have reported the primary outcomes from the study.[12,13]
Placental transfer of Ocrevus and impact on B-cell levels in newborns
All 35 pregnancies resulted in fullterm births, with all babies showing normal weight, length, and head circumference.[12]
- All infant (100%, 34/34) B-cell levels were above the LLN at Week 6, indicating that exposure to Ocrevus did not result in infant B-cell depletion.[12]
- Ocrevus was undetectable in most umbilical cord serum at birth (94%, 33/35) and infant serum at Week 6 of life (97%, 32/33), indicating minimal placental transfer and low exposure in utero.[12]
Maternal humoral responses to vaccines
All median antibody titers to immunizations measured in pregnant women during the third trimester were above seroprotective levels.[13] Most pregnant women had positive humoral responses against clinically relevant pathogens.
Adverse events in mothers
Adverse events (AEs) occurred in 91% of women and were consistent with the established Ocrevus safety profile and typical for pregnancy, delivery, and postpartum.
- Infections or infestations occurred in 54%; most common were COVID-19 (17%) and nasopharyngitis (11%).
- Serious AEs occurred in 17%.
- Treatment-related AEs occurred in 26%
- AEs that led to treatment withdrawal occurred in 3%.
Adverse events in infants
Adverse events occurred in 74% of infants, and most were mild (66%) to moderate (46%).
- Infections occurred in 46% and were typical of infancy
- most common were nasopharyngitis (17%), ear infection (14%), and bronchitis (14%).
- Serious AEs occurred in 11% and were not considered related to Ocrevus
- all resolved including Grade 3 infection and respiratory failure in 1 infant, heart rate decrease, RSV infection, and 1 Grade 4 cardiopulmonary failure.
Reports from registries and observational studies
MSBaseRegistry
Yeh et al. conducted a real-world, retrospective cohort study of ARR in the 1,744 women with 2,009 term and pre-term pregnancies documented in the international MSBase Registry up to July 1, 2023.[14,15] Eligible patients were ≥18 years old with a diagnosis of RRMS or CIS at conception and using Ocrevus (n=73), rituximab (n=24), natalizumab, dimethyl fumarate, or low-efficacy (interferon-beta or glatiramer acetate) as the most recent DMT prior to conception.The women were followed up to 6 months after pregnancy end. In the Ocrevus group, no relapses were observed during pregnancy. In the postpartum period, the Ocrevus group experienced 3 relapses, with the lowest ARR in comparison to the other DMTs (0.09 versus 0.10 to 0.74).
Roche global safety database and Canadian Roche Patient Support Program (COMPASS)
Krysko et al. conducted a real-world observational study of MS disease activity among 208 women treated with Ocrevus in COMPASS (RRMS: 205, PPMS: 3) who had pregnancies with a live birth.[16] Postpartum, 169 (81.3%) women resumed Ocrevus at a median of 2.1 months post-delivery. There were 690 questionnaires completed to assess patient-reported MS disease activity; 52 (7.5%) reported MS disease worsening. Two (1.0%) relapses were reported pre-pregnancy, no relapses occurred during pregnancy and 4 (1.9%) relapses were reported postpartum. AEs were in line with the Ocrevus safety profile.
Gitman et al. evaluated pregnancy and fetal outcomes in 107 spontaneously reported or non-interventional cases of maternal exposure to Ocrevus obtained from the Roche global safety database and the Canadian Roche patient support program (COMPASS).[17] The study included 107 women with MS in Canada who had at least 1 dose of Ocrevus and reported a pregnancy exposure to Roche. Ongoing pregnancies and paternal exposure to Ocrevus were excluded from the analysis.
- Of the 107 maternal exposure cases, 50 (46.7%) had no in utero exposure , 37 (34.6%) had in utero exposure, and 20 (18.7%) had unknown exposure.
- out of the 65 cases with known outcomes, 47 live births were reported, of which 21 were full-term, 4 cases were preterm (2 sets of twins), and 22 were unknown.
- One infant, who was exposed to Ocrevus in the first trimester, was born with polydactyly at gestational Week 37; however, multiple confounders, including family history of polydactyly, were noted.
- Additionally, there were 13 spontaneous abortions, 3 ectopic pregnancies, and 2 elective terminations reported.
Infant exposure to Ocrevus through breastfeeding
Roche global safety database
As of March 2024, 575 infants had 1-year follow-up data available.[2] One hundred fifty-eight infants were exposed to Ocrevus through breastfeeding, while 417 infants had no exposure through breastfeeding. Among the babies with breastfeeding exposure to Ocrevus, 51 (8.9%) were exclusively exposed via breastfeeding. Infant outcomes remain limited due to incomplete reports (up to 95.6% missing information).[2]
SOPRANINO study
Study design
SOPRANINO is a prospective, multicenter, open-label study in women with CIS or MS, evaluating the pharmacokinetics of Ocrevus in the breast milk of lactating women and the resultant exposure and pharmacodynamic effects in the infant.[10,18] The study will enrol at least 20 women who delivered a term infant and plan to breastfeed for at least 60 days after the first postpartum Ocrevus infusion.
The co-primary endpoints of the study are
- proportion of infants with B-cell levels below the LLN, measured 30 days after the mother’s first postpartum Ocrevus infusion, and
- estimated average daily infant dose over 60 days after the mother’s first postpartum Ocrevus infusion.
Additional endpoints will assess immune responses, infant growth and development, and adverse events in the mother and infant.
Results
The primary analysis was presented in breastfed infants 30 days after maternal infusion for 13 mothers with RRMS.[19] Among the 13 infant and mother pairs enrolled:
- Ocrevus levels in breast milk were negligible (ADID: 45.1 μg; average RID: 0.3%; maximum RID: 0.8%)
- Ocrevus was undetectable in infant serum; 9 out of 9 infants had serum Ocrevus levels below the lower limit of quantification (156 ng/mL).
All infants had B-cell levels within normal ranges (n=10 analyzed) as defined by Borriello et al.[20]
Adverse events
Eleven infants (85%) had at least 1 AE (9 had grades 1-2; one had grade 3 bronchiolitis, resolved). Ten infants (77%) had infections, consistent with common childhood diseases in the first year of life, all of which resolved. The most common infections were:
- COVID-19 (31%)
- ear infection (23%),
- bronchiolitis (15%)
- nasopharyngitis (15%)
No serious AEs were reported.
Prospective Multicenter Study
Anderson et al. collected breast milk samples from 57 women (59 pregnancies) with MS or NMOSD, who were treated across 10 different MS Centers.[21] Enrolled women and their newborns were followed up for up to 12 months postpartum or 90 days post-infusion. The patients received Ocrevus (n=33) or rituximab (n=26) while breastfeeding, and breast milk samples were collected before infusion and serially for up to 90 days after the infusion.
The earliest timepoint of Ocrevus administration was a few days after birth,and the median timepoint was 4.3 months postpartum. Twenty-four women continued to breastfeed after infusion of Ocrevus. The median duration of breastfeeding post-infusion was 6.4 months (range: 0.3-11.7).[21]
Concentration of Ocrevus in breast milk
The median average concentration of Ocrevus in breast milk was low at 0.08 µg/mL (range: 0.05-0.4) and the median peak concentration was 0.3 µg/mL (range 0.2-0.5) , occurring mostly 1 to 7 days post-infusion. Based on the average concentration, the RID was 0.1% (range 0.07-0.7). Ocrevus was virtually undetectable in breast milk by 90 days post-infusion.[21]
Infant outcomes
At 8-12 months, there was no significant difference in growth or development, between breastfed and non-breastfed infants (p>0.05).
- 53 of 55 infants completed their routine vaccinations (3 mothers deferred rotavirus vaccination).
- Four breastfed infants (between 2.1 to 6.2 months-old) evaluated for IgG and CD19 levels in the peripheral blood, had levels within the normal range.
- With the exception of common minor infections, the infants with clinical records available (n=56) did not experience any frequent or severe infections.
German MS and Pregnancy Registry
Ciplea et al. reported on 23 patients from the German MS and Pregnancy Registry (DMSKW) who received various monoclonal antibodies, including 3 who received Ocrevus.[22] Only 1 of the women received Ocrevus during pregnancy in the second trimester, and the other two received Ocrevus at 20 and 194 days postpartum, respectively. In the infant of the mother who received Ocrevus in the second trimester, the B-cell levels were decreased at birth (CD19: 339/mcL), but normalized during exposed breastfeeding at 79 days postpartum. The other 2 babies had normal B-cell levels during breastfeeding.
References
- Vukusic S, Bove R, Dobson R, et al. Pregnancy and Infant Outcomes in Women With Multiple Sclerosis Treated With Ocrelizumab. Neurol Neuroimmunol Neuroinflamm 2025;12:e200349. https://www.ncbi.nlm.nih.gov/pubmed/39689270
- Dobson R, Vukusic S, Bove R, et al. Pregnancy and Infant Outcomes in Women with Multiple Sclerosis Receiving Ocrelizumab: Analysis of Approximately 4,000 Pregnancies to Date. Presented at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis in Copenhagen, Denmark; September 18-20, 2024. ECTRIMS Poster #P085.
- Roche Internal Safety Report (Accessed on 7th October 2024).
- Lopez-Leon S, Geissbühler Y, Sabidó M, et al. A systematic review and meta-analyses of pregnancy and fetal outcomes in women with multiple sclerosis: a contribution from the IMI2 ConcePTION project. J Neurol 2020;267:2721-2731. https://www.ncbi.nlm.nih.gov/pubmed/32444984
- Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Weekly Report. January 11, 2008. January 11, 2008. Available at thttps://www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a2.htm.
- Andersen JB, Sellebjerg F, Magyari M. Pregnancy outcomes after early fetal exposure to injectable first-line treatments, dimethyl fumarate, or natalizumab in Danish women with multiple sclerosis. Eur J Neurol 2023;30:162-171. https://www.ncbi.nlm.nih.gov/pubmed/36098960
- MacDonald SC, McElrath TF, Hernández-Díaz S. Pregnancy Outcomes in Women With Multiple Sclerosis. Am J Epidemiol 2019;188:57-66. https://www.ncbi.nlm.nih.gov/pubmed/30165561
- Khan E, Kagzi Y, Elkhooly M, et al. Disease modifying therapy and pregnancy outcomes in multiple sclerosis: A systematic review and meta-analysis. J Neuroimmunol 2023;383:578178. https://www.ncbi.nlm.nih.gov/pubmed/37672841
- Vukusic S, Perrin Ross A, Oreja-Guevara C, et al. Disease Activity Before, During and After Pregnancy in Women with MS Receiving Ocrelizumab: An Integrated Analysis From 13 Interventional Clinical Trials. Presented at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis in Copenhagen, Denmark; September 18-20, 2024. ECRIMS Poster #P591.
- Bove R, Hellwig K, Pasquarelli N, et al. Ocrelizumab during pregnancy and lactation: Rationale and design of the MINORE and SOPRANINO studies in women with MS and their infants. Mult Scler Relat Disord 2022;64:103963. https://www.ncbi.nlm.nih.gov/pubmed/35753176
- Hellwig K, Pasquarelli N, Borriello F, et al. Rationale and design of a Phase 4 study exploring B-cell levels and immune responses in infants born to women with MS who were exposed to ocrelizumab up to 6 months before or during the first trimester of pregnancy (the MINORE study). Presented at the European Committee for Treatment and Research in Multiple Sclerosis in virtual; October 13-15, 2021. ECTRIMS Poster. https://www.ectrims-congress.eu/2021.html
- Hellwig K, Bove R, Oreja-Guevara C, et al. B-Cell Levels and Placental Transfer in Infants Potentially Exposed to Ocrelizumab During Pregnancy: Primary Analysis of the Prospective Multicentre, Open- Label Phase IV MINORE Study. Presented at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis in Copenhagen, Denmark; September 18-20, 2024. ECTRIMS Poster #P087.
- Hellwig K, Bove R, Oreja-Guevara B, et al. Infant B-Cell Levels, Ocrelizumab Placental Transfer and Maternal Humoral Responses During Pregnancy: Primary Analysis of the Prospective, Multicenter, Open-Label Phase IV MINORE Study. Presented at the Americas Committee for Treatment and Research in Multiple Sclerosis in West Palm Beach, Florida, USA; February 27 - March 1, 2025. ACTRIMS Poster #P106.
- Yeh W, VanderWalt A, Kermode A, et al. Disease activity during pregnancy and postpartum in women with MS receiving ocrelizumab in a real-world cohort. Presented at the Congress of the European Academy of Neurology in Vienna, Austria; June 25-28, 2022. EAN Oral presentation. https://www.ean.org/congress2022
- Yeh WZ, Van Der Walt A, Skibina OG, et al. Disease Activity in Pregnant and Postpartum Women With Multiple Sclerosis Receiving Ocrelizumab or Other Disease-Modifying Therapies. Neurol Neuroimmunol Neuroinflamm 2024;11:e200328. https://www.ncbi.nlm.nih.gov/pubmed/39442037
- Krysko K, Gitman V, Spasojevic S, et al. Real-World Evaluation of Multiple Sclerosis Pregnancy-Related Disease Activity in Women Treated With Ocrelizumab: Insights from a Canadian Patient Support Program. Presented at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis in Copenhagen, Denmark; September 18-20, 2024. ECTRIMS Poster #P1240.
- Gitman V, Stavropoulos A, Saenz V, et al. Pregnancy outcomes of women with multiple sclerosis treated with ocrelizumab in Canada: A descriptive analysis of real-world data. Mult Scler Relat Disord 2022;62:103792. https://www.ncbi.nlm.nih.gov/pubmed/35452964
- Bove R, Pasquarelli N, Borriello F, et al. B-cell levels and immunity in breastfed infants of women with MS treated with ocrelizumab: design of a Phase 4 study (SOPRANINO). Presented at the European Committee for Treatment and Research in Multiple Sclerosis in virtual; October 13-15, 2021. ECTRIMS Poster #P686. https://www.ectrims-congress.eu/2021.html
- Bove R, Oreja-Guevara C, Hellwig K, et al. B-Cell Levels and Breastmilk Transfer in Infants of Lactating Women With Multiple Sclerosis Treated With Ocrelizumab: Primary Results of the Prospective Multicentre, Open-Label Phase IV Study SOPRANINO. Presented at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis in Copenhagen, Denmark; September 18-20, 2024. ECTRIMS Oral presentation #O039.
- Borriello F, Pasquarelli N, Law L, et al. Normal B-cell ranges in infants: A systematic review and meta-analysis. J Allergy Clin Immunol 2022;150:1216-1224. https://www.ncbi.nlm.nih.gov/pubmed/35728653
- Anderson A, Rowles W, Poole S, et al. Anti-CD20 monoclonal antibody therapy in postpartum women with neurological conditions. Ann Clin Transl Neurol 2023;10:2053-2064. https://www.ncbi.nlm.nih.gov/pubmed/37675826
- Ciplea AI, Langer-Gould A, de VA, et al. Monoclonal antibody treatment during pregnancy and/or lactation in women with MS or neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. E-pub Date: July 2020. DOI # 10.1212/NXI.0000000000000723. https://www.ncbi.nlm.nih.gov/pubmed/32327455
Medinfo
Need to contact Roche?
Request Product Information
Request Product Information
Ask us a question and request information about Roche products or services.
Report a potential side effect
Report a potential side effect
If you have experienced potential side effects with a Roche product you can report it here.
Report a potential product defect
Report a potential product defect
If you suspect a potential defect or a Roche product has not met your expectations you can report it here.
Request temperature stability assessment
Request temperature stability assessment
Request an assessment if your product was stored outside the recommended temperature range.
