Perjeta Dosing and Administration Recommendations
This article responds to your request for information on Perjeta® (pertuzumab) intravenous infusion and dosing and administration recommendations.
Please refer to the locally approved dosing information provided in the Perjeta package insert or prescribing information. Any deviation from this information is considered off-label and any treatment decisions based on such deviations are the full responsibility of the prescribing physician.
Last updated March 27, 2025
Summary
- There are no recommendations for administering premedications in the Perjeta prescribing information.
- The recommended loading dose of Perjeta is 840 mg, followed every 3 weeks thereafter by a maintenance dose of 420 mg.
- If a patient experiences a delayed or missed dose, they may need to be administered the loading doses of Perjeta and Herceptin® (trastuzumab) again.
- Observe patients during Perjeta administration and for 60 minutes following the administration of a loading dose, or 30 minutes following a maintenance dose.
Administering premedication
There are no recommendations for administering premedications in the Perjeta prescribing information.[1]
Premedication protocol in the pivotal studies
Pivotal clinical studies, including CLEOPATRA and APHINITY, did not require that patients were premedicated prior to receiving Perjeta.[2,3] While the studies did not require premedications, they were permitted to be used in line with local clinical practice. This included, but was not limited to
- paracetamol and other analgesics
- diphenhydramine, chlorpheniramine or other anti-histamines, and
- anti-emetics.
Loading and maintenance dose
The recommended loading dose of Perjeta is 840 mg administered as a 60 minute intravenous infusion, followed by a maintenance dose of 420 mg administered over a period of 30 to 60 minutes every 3 weeks thereafter.[1] Both loading and maintenance dose is irrespective of patient body weight.[1]
Combination treatment with Herceptin
Perjeta is indicated in combination with Herceptin.[1] Perjeta and Herceptin should be administered sequentially and can be given in any order. When administered with Perjeta, follow a 3-weekly schedule for Herceptin administered either as [1]
- Herceptin IV - an initial dose of 8 mg/kg followed every 3 weeks thereafter by 6 mg/kg, by intravenous infusion
- Herceptin SC - 600mg irrespective of the patient’s body weight every 3 weeks, by subcutaneous injection
Discontinue Perjeta if Herceptin treatment is discontinued.[1]
Dosing following delayed or missed treatment
If a patient experiences a delayed or missed dose of Perjeta, the next dose should be administered as soon as possible.[1] Do not wait until the next planned dose.
In clinical studies, if either Perjeta or Herceptin had to be delayed by a day or more, both treatments were required to be delayed for the same timeframe.[2,3]
Depending on the time between two sequential doses, the patient may need to be administered the loading dose again.[1] Refer to Table 1 for recommendations on when re-loading doses may be required for Perjeta and Herceptin.
Table 1. Recommendations regarding delayed or missed doses
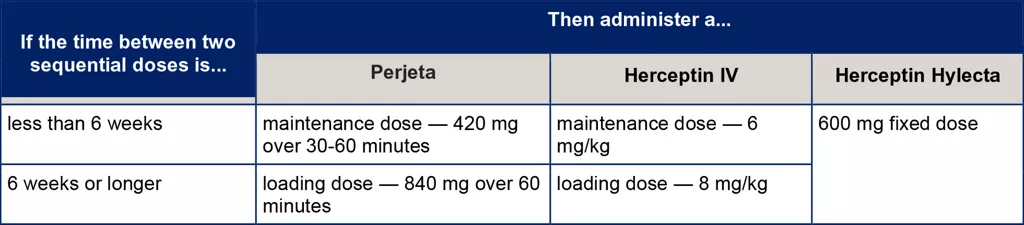
Once the patient has received either a loading or maintenance dose following the delay, the 3 weekly interval should be based on the latest dose, rather than the prior schedule.[1]
Dose delay example
A patient receives a maintenance dose of 420 mg Perjeta on 1st January. Their next dose is due on 22nd January, but because of travel plans, they are unable to receive it until 29th January (a 4 week interval). They should not receive their Herceptin IV dose on 22nd of January.
On 29th January, they should receive the maintenance doses of Perjeta 420 mg over 30 to 60 minutes and Herceptin 6 mg/kg IV.
Originally, they should have been due their following doses on 12th February, or 3 weeks from 22nd January. However, based on their new schedule, they will now be due to receive their next maintenance doses on 19th February, or 3 weeks from 29th January, and then every 3 weeks thereafter.
Switching from Phesgo
The Phase 2 PHranceSCa study evaluated patient preference for Phesgo, a fixed-dose subcutaneous injection combination of pertuzumab, trastuzumab and hyaluronidase, compared with IV Perjeta and IV Herceptin in the treatment of early HER2 positive breast cancer in the adjuvant setting.[4] In one arm of the study patients were administered 3 cycles of Phesgo every 3 weeks and then switched to receive 3 cycles of IV Perjeta and Herceptin every 3 weeks.
The initial dose of IV Perjeta after the switch from Phesgo was given at the maintenance dose of 420 mg if it was less than 6 weeks since their last treatment. If it had been 6 weeks or more since their last treatment, a loading dose of 840 mg was administered.[4]
Recommended observation times
Perjeta has been associated with infusion-related reactions, including events with fatal outcomes.[1] It is recommended to closely observe the patient during and for a set period following Perjeta infusions:
- First infusion — 60 minutes
- Subsequent infusions — 30 minutes
Complete the observation period prior to any subsequent dose of Herceptin or chemotherapy.[1]
References
- Roche Internal Regulatory Report. Accessed 21 June 2023.
- Protocol CLEOPATRA Trial: Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. December 8, 2011. Available at http://www.nejm.org/doi/suppl/10.1056/NEJMoa1113216/suppl_file/nejmoa1113216_protocol.pdf. Accessed on February 27, 2017.
- Protocol for APHINITY Trial: Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. July 21, 2017. Available at http://www.nejm.org/doi/suppl/10.1056/NEJMoa1703643/suppl_file/nejmoa1703643_protocol.pdf. Accessed on June 5, 2017.
- O'Shaughnessy J, Sousa S, Cruz J, et al. Preference for the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with HER2-positive early breast cancer (PHranceSCa): A randomised, open-label phase II study. Eur J Cancer 2021;152:223-232. https://www.ncbi.nlm.nih.gov/pubmed/34147014
Medinfo
Need to contact Roche?
Request Product Information
Request Product Information
Ask us a question and request information about Roche products or services.
Report a potential side effect
Report a potential side effect
If you have experienced potential side effects with a Roche product you can report it here.
Report a potential product defect
Report a potential product defect
If you suspect a potential defect or a Roche product has not met your expectations you can report it here.
Request temperature stability assessment
Request temperature stability assessment
Request an assessment if your product was stored outside the recommended temperature range.
