
Lupus Nephritis
Introduction
Lupus nephritis (LN) is one of the most severe organ manifestations of systemic lupus erythematosus (SLE), an autoimmune disease characterised by loss of self-tolerance and proliferation of autoantibodies.1,2 LN is characterised by the formation and deposition of immune complexes that lead to inflammation and fibrosis within the glomeruli and tubulointerstitium of the kidneys.3,4 Flares of disease activity result in progressive nephron loss reflected in a declining glomerular filtration rate (see Figure 1)5 and substantially increased risk of end-stage kidney disease (ESKD) and death.3,6
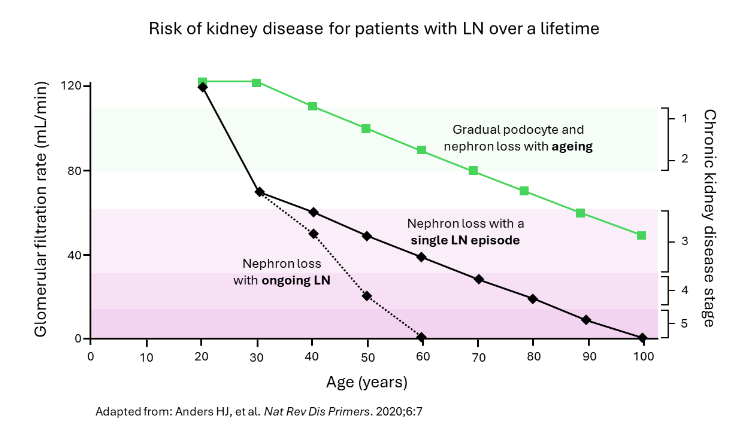 Figure 1: Risk of kidney disease for patients with LN over a lifetime.
Figure 1: Risk of kidney disease for patients with LN over a lifetime.
Prevalence
The prevalence of SLE varies by geographic region, and it has been reported to affect more than 3.4 million people worldwide.1,7 With approximately 50% of patients with SLE developing LN,3,5,8 the estimated number of people living with LN amounts to 1.4 million.1 Approximately 90% of people living with SLE are women.1 The prevalence of LN in SLE patients is greater in African American (34–51%), Hispanic (31–49%), and Asian (33–82%) individuals than in Caucasians (14–23%).5
Disease Burden
LN is associated with mortality rates that are 6 times higher than those of the general population.6
Approximately 50% of SLE patients will develop LN within 5 years of SLE diagnosis,3,5,8 and up to 30% of patients with LN will develop ESKD within 10 years of diagnosis, requiring kidney replacement therapy (dialysis or transplantation), despite treatment with current available therapies.3,8,9 Overall, 7-31% of patients have LN at SLE diagnosis;10 whilst women of childbearing age are most predominantly affected by SLE, men with SLE are at a greater risk of developing LN.3
Unmet Medical Need
Currently, there is no cure for LN, and management of the disease relies on controlling inflammation, suppressing the immune system, and the use of other supportive management.11-13 In addition to lifestyle modifications, drug treatments can include antihypertensives, steroids, antimalarials, immunosuppressants, and biologic therapies.11-13 With current treatments, only approximately 30% of patients achieve a clinical response; relapses are common, and concerns about drug-related toxicity persist.5,14
Current guidelines are a valuable source of peer-reviewed information on the management and treatment of patients with LN.11-13
Patients’ Needs
Figure 2 depicts patient-perceived benefits for people living with LN as well as the societal impact of the disease.15-20
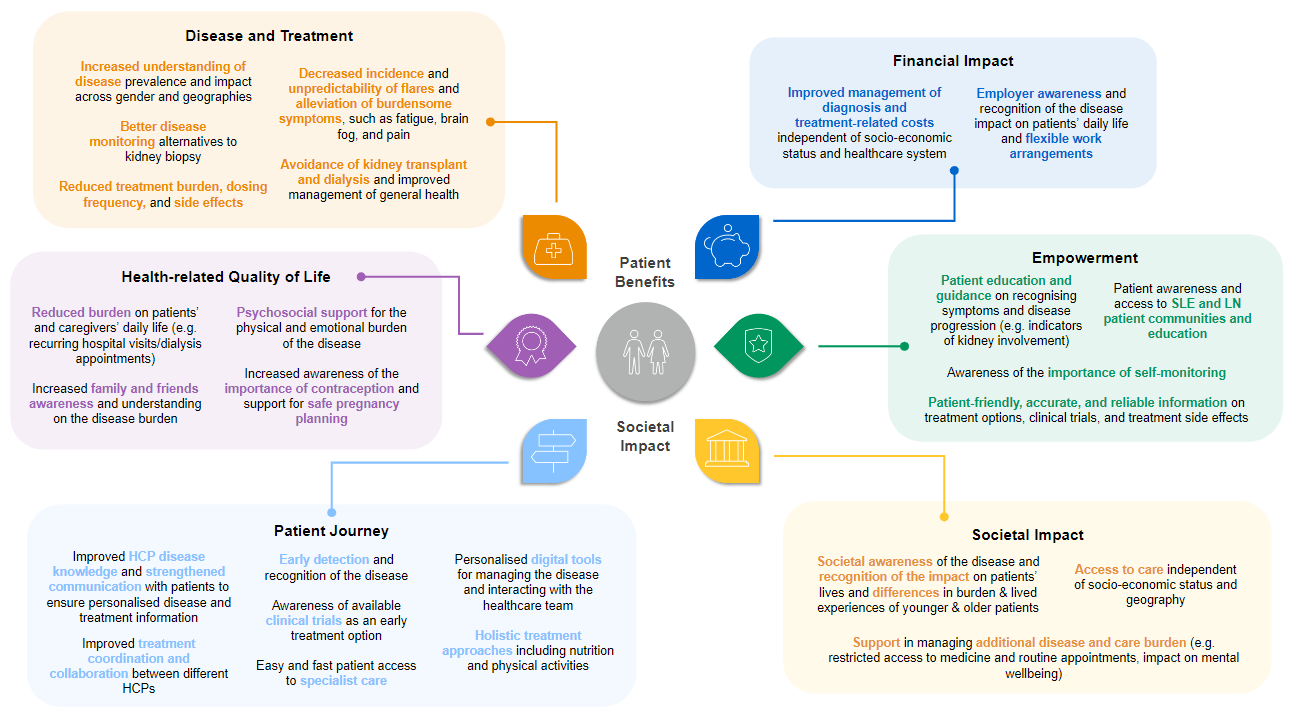 Figure 2: Patient benefits and societal impact of SLE and LN.15-20
Figure 2: Patient benefits and societal impact of SLE and LN.15-20
Peers’ Perspectives
On B-cell depletion
Prof. Brad Rovin
On the need to act with urgency
Dr. Ioannis Parodis
On steroids
Prof. Brad Rovin
On steroids
Prof. Liz Lightstone
On B-cell depletion
Dr. Juan Mejia-Vilet
On biopsies
Prof. Brad Rovin and
Dr. Juan Mejia-Vilet
On biomarkers
Prof. Brad Rovin and
Dr. Juan Mejia-Vilet
References
- Tsokos G, et al. Nat Rev Rheumatol. 2016;12:716–730.
- Hoi A, et al. The Lancet 2024;403:2326–38.
- Parikh SV, et al. Am J Kidney Dis 2020;76:265–281.
- Mohan C, et al. Nat Rev Nephrol 2023;19:491–508.
- Anders HJ, et al. Nat Rev Dis Primers. 2020;6:7.
- Hocaoglu M, et al. Arthritis Rheumatol 2023;75:567–573.
- Tian J, et al. Ann Rheum Dis 2023:82:351–56.
- Bechler KK, et al. AMIA Annu Symp Proc. 2023;2022:221–230.
- Mok CC, et al. Nat Rev Rheumatol 2023;19:227–238.
- Mahajan A, et al. Lupus 2020;29:1011–1020.
- Kidney Disease: Improving Global Outcomes Lupus Nephritis Work Group, KDIGO 2024 Clinical Practice Guideline for glomerulonephritis. Kidney Int Supplements 2012, 2, 221–232.
- Kidney Disease: Improving Global Outcomes (KDIGO) Lupus Nephritis Work Group. Kidney Int. 2024;105:S1–S69.
- Fanouriakis A, et al. Ann Rheum Dis. 2024;83:15–29.
- Parodis I, et al. Autoimmun Rev 2024;23:103418.
- Lupus and Allied Diseases Association, Inc, Lupus Foundation of America, and Lupus Research Alliance. Lupus: Patient Voices Report on Externally-led Patient-Focused Drug Development Meeting. 2018. https://s3.amazonaws.com/stg.files.lupus.org/public/images/Advocacy/Documents/Lupus%20-%20Patient%20Voices%20Report.pdf. Accessed August 2024.
- Lupus Europe. Patient Panel 2 project report. 2016. https://www.lupus-europe.org/wp-content/uploads/2018/10/patient-panel-II-report-Short-final.pdf. Accessed August 2024.
- Lupus Europe. Patient Panel 3 project report. 2018. https://www.lupus-europe.org/wp-content/uploads/2018/10/patient-panel-III-report-Final.pdf. Accessed August 2024.
- Lupus Europe. Lupus Europe Convention 2023 report. 2023. https://www.lupus-europe.org/wp-content/uploads/2023/07/Convention-Report-2023.pdf. Accessed August 2024.
- Cornet A, et al. Lupus Sci Med. 2021;8:e000469.
- Roche, Data on file.
