
Systemic Lupus Erythematosus
Introduction
Systemic lupus erythematosus (SLE), also commonly called lupus, is a systemic autoimmune disease characterised by loss of self-tolerance and proliferation of autoantibodies that cause immune-complex deposition and subsequent tissue damage.1 The disease shows diverse clinical manifestations.1 The most common organ-threatening manifestation of SLE is lupus nephritis (LN), characterised by the formation and deposition of immune complexes leading to inflammation and fibrosis within the glomeruli and tubulointerstitium of the kidneys.2,3
B-cell dysfunction and pathogenic autoantibody formation have been implicated in the pathogenesis of SLE.4,5 Autoreactive, pathogenic B cells recognise self-antigens, produce autoantibodies, secrete proinflammatory cytokines, and participate in T-cell co-stimulation.6 In people living with LN, B cells infiltrate the kidneys, and it has been shown that leukocyte-rich tubulointerstitial infiltrates are associated with greater risk of progression to renal failure.3
Prevalence
SLE affects more than 3.4 million people worldwide, with a female predominance (90%) and considerable variability by geographic region, being more prevalent in people of non-Caucasian background.2,7,8 The prevalence of SLE per 100,000 people is estimated to be 48–366.6 in North America, 29.3–210 in Europe, 24.3–126.3 in South America, 20.6–103 in Asia, 13–52 in Australasia, and 601.3–7713.5 in Africa.9 The prevalence estimates in Africa appear significantly higher than those in other regions, but these are unlikely to reflect the true population prevalence as they are based on hospital and clinic samples rather than population- and registry-based studies.9
Approximately 15–20% of SLE patients are diagnosed before the age of 18 years, with an average age of 12–14 years at onset of childhood-onset SLE.10,11 The typical age of SLE diagnosis can vary significantly, with an average of 33.6 years and a range of 19.3–47.9 years.12
Disease Burden
SLE exhibits diverse and often serious clinical manifestations (see Figure 1).1 In addition, patients with SLE are at an increased risk of developing ischemic heart disease or stroke.13 Approximately 80 to 90% of patients with SLE report fatigue to be the most debilitating symptom, affecting work, leisure, and overall quality of life.13 Relative to adults, children with SLE are more likely to have active disease, especially renal complications.12,14
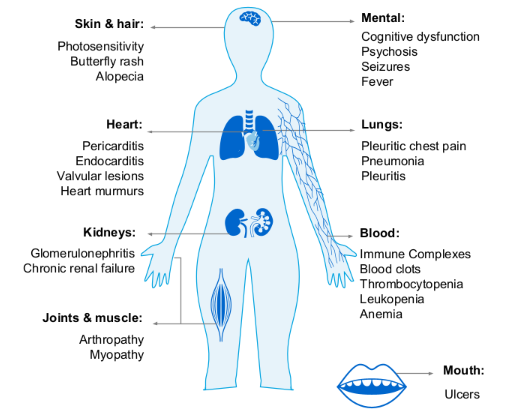
Figure 1: Possible manifestations of SLE.
Unmet Medical Need
Currently, there is no cure for SLE, and management of the disease is dependent on controlling inflammation, suppressing the immune system, and other supportive management.15,16 Drug treatments can include antihypertensives, corticosteroids, antimalarials, immunosuppressants, and biologic therapies.15,16 The treatments themselves add to the disease burden, notably the toxicities associated with cumulative steroid doses.15 The European Alliance of Associations for Rheumatology (EULAR) and Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend reduced corticosteroid regimens and the early use of disease-modifying antirheumatic drugs and biologics as steroid-sparing agents for the treatment of SLE.15,16 EULAR recommendations include antimalarials, glucocorticoids, and immunomodulating and immunosuppressive therapies.16 In patients with organ- or life-threatening disease, cyclophosphamide should be considered.16 Up to 25% of patients receiving currently approved therapies may experience relapse or refractory SLE,17 highlighting the need for more efficacious treatment options.
Patients’ Needs
Roche and the patient community worked together to review the literature and characterise the unmet needs of patients with SLE and LN (see Figure 2).18-23
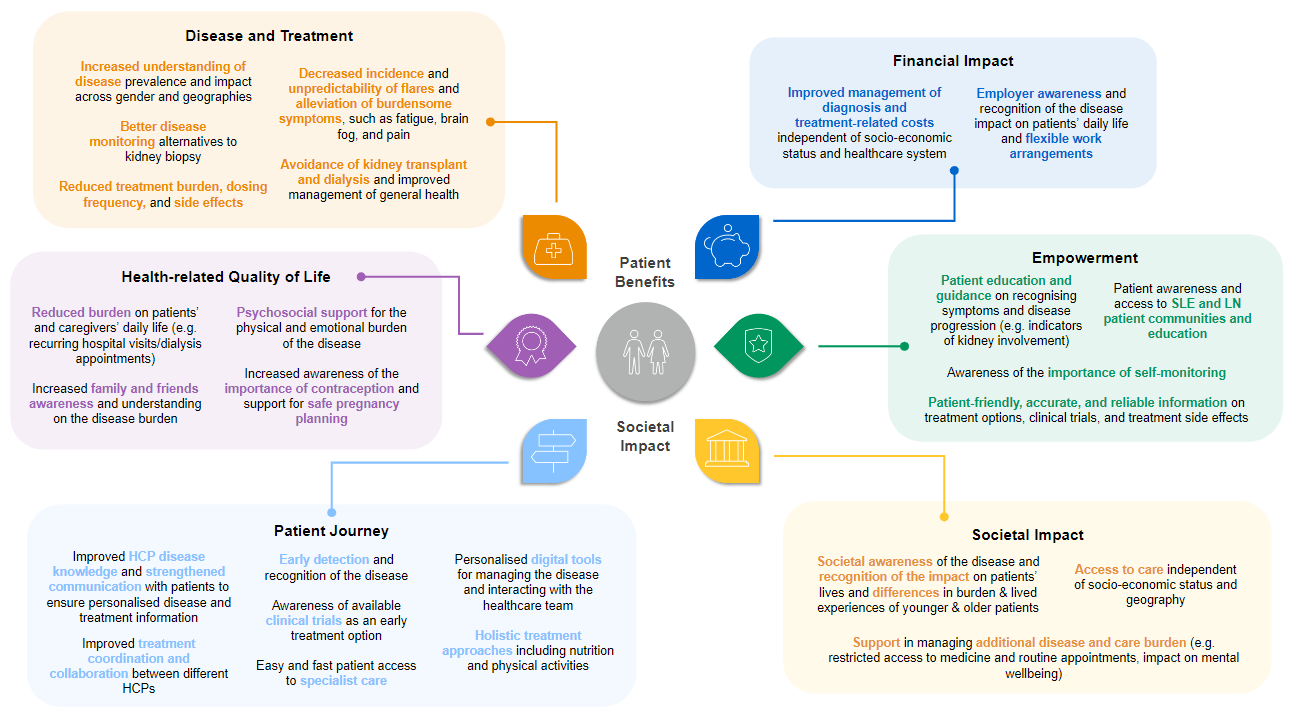 Figure 2: Patient benefits and societal impact of SLE and LN.
Figure 2: Patient benefits and societal impact of SLE and LN.
Learn More
Understanding patients’ needs and the biology of SLE is pivotal in developing therapies. Targeting B cells, which are widely recognised as a key driver in SLE pathogenesis, is believed to be a promising therapeutic approach, with B-cell therapies being approved and continuously under development.4,15
References
- Tsokos G, et al. Nat Rev Rheumatol. 2016;12:716-730.
- Parikh SV, et al. Am J Kidney Dis. 2020;76:265-281.
- Mohan C, et al. Nat Rev Nephrol. 2023;19:491-508.
- Nashi E, et al. Int J Biochem Cell Biol. 2010;42:543-550.
- Yap DYH, Chan TM. Int J Mol Sci. 2019;20:6231.
- Stensland ZC, et al. Biomedicines. 2021;9,83.
- Tian J, et al. Ann Rheum Dis. 2023;82:351-356.
- Hoi A, et al. Lancet. 2024;403:2326-2338.
- Barber MRW, et al. Nat Rev Rheumatol. 2021;17:515-532.
- Massias J, et al. Lupus. 2020;29:474-481.
- Thakral A, Klein-Gitelman MS. Rheumatol Ther. 2016;3:209-219.
- Sassi RH, et al. Clin Rheumatol. 2017;36:89-95.
- Bakshi J, et al. Clinic Rev Allerg Immunol. 2018;55:352-367.
- Sato V, et al. Lupus. 2012;21:978-983.
- Kidney Disease: Improving Global Outcomes (KDIGO) Lupus Nephritis Work Group. Kidney Int. 2024;105:S1-S69.
- Boumpas D. EULAR 2023 Congress [EULAR-Recommendations on the management of lupus].
- Menez SP, et al. Rev Recent Clin Trials. 2018;13:105-113.
- Lupus and Allied Diseases Association, Inc., Lupus Foundation of America, and Lupus Research Alliance. Lupus: Patient Voices Report on Externally-led Patient-Focused Drug Development Meeting. 2018. https://s3.amazonaws.com/stg.files.lupus.org/public/images/Advocacy/Documents/Lupus%20-%20Patient%20Voices%20Report.pdf. Accessed August 2024.
- Lupus Europe. Patient Panel 2 project report. 2016. https://www.lupus-europe.org/wp-content/uploads/2018/10/patient-panel-II-report-Short-final.pdf. Accessed August 2024.
- Lupus Europe. Patient Panel 3 project report. 2018. https://www.lupus-europe.org/wp-content/uploads/2018/10/patient-panel-III-report-Final.pdf. Accessed August 2024.
- Lupus Europe. Lupus Europe Convention 2023 report. 2023. https://www.lupus-europe.org/wp-content/uploads/2023/07/Convention-Report-2023.pdf. Accessed August 2024.
- Cornet A, et al. Lupus Sci Med. 2021;8:e000469.
- Roche, Data on file.
