
Tumor agnostic in Oncology
Molecular landscape of NTRK fusions in tumor agnostic
How to test for NTRK fusions?
ESMO proposal: NTRK testing1
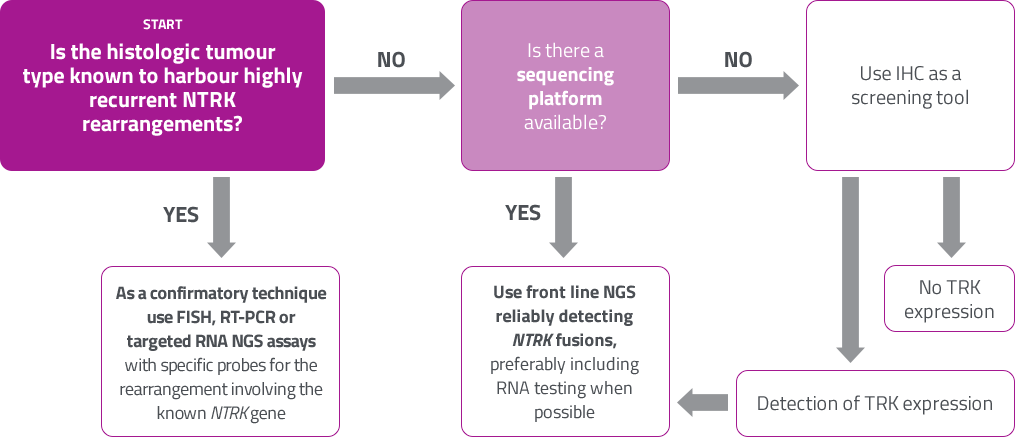
It is important to ensure that the diagnostic test covers NTRK 1, 2, 3 fusion genes and is validated with appropriate reference standards.
[Figure adapted from Marchio C. et al , 2019.]
High-quality molecular testing is needed to uncover NTRK fusion+ cancer2,3,4
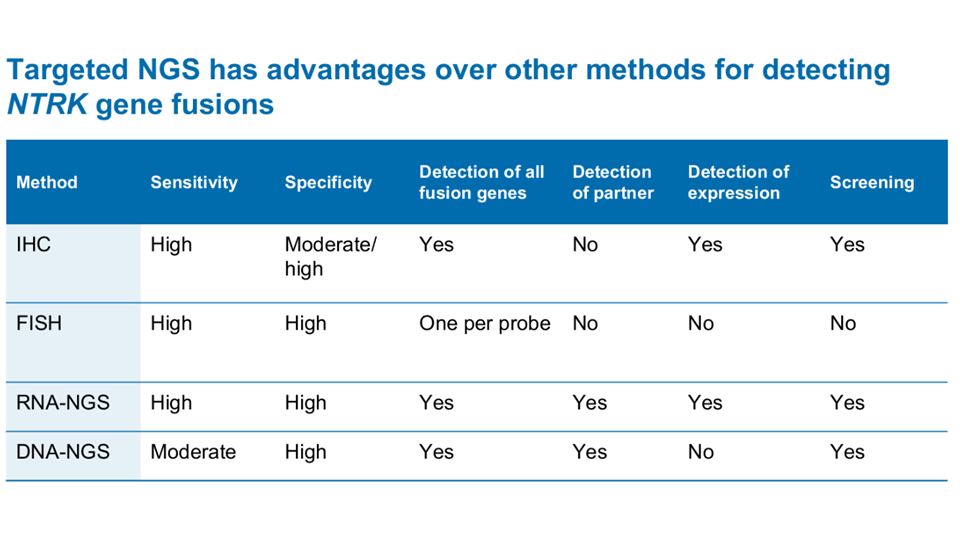
Marchio, et al. Ann Oncol 2019
References
1. Marchio C. et al. Ann Oncol 2019;30:1417–1427.
2. Vaishnavi A, Le AT, Doebele RC. Cancer Discov 2015;5:25–34.
3. Murphy DA, et al. Appl Immunohisochem Mol Morphol 2017;25:513–523.
4. Rogers TM, et al. Sci Rep 2017;7:1–8.
