
Retinal Disease Hub
Overview
In the retina, under pathologic conditions, an angiogenic switch occurs, shifting the balance from anti- to pro-angiogenic factors, including the upregulation of Ang-2 as well as VEGF.1
Clinical relevance
Ang-2 levels in human vitreous samples
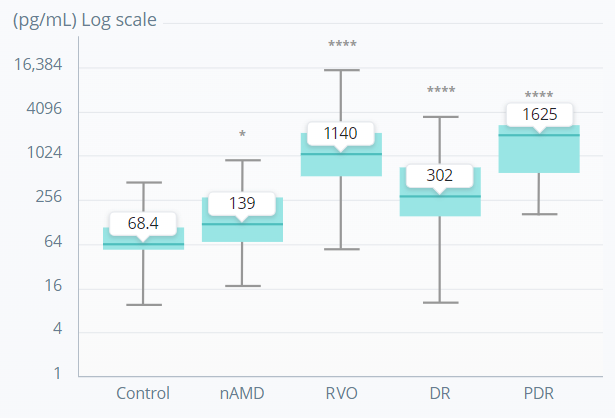
*p<0.05; ****p<0.0001.
A nonparametric Kruskal–Wallis analysis followed by Dunn’s method for multiple comparisons was used to show significant differences of the groups to control.
Adapted from Regula JT, et al. EMBO Mol Med. 2016;8(11):1265–88.
Ang-2 levels are increased in the vitreous of patients with retinal or choroidal vascular diseases, including AMD, DR, and RVO, supporting a role for Ang-2–Tie2 signalling in these pathologic conditions.3,4
Preclinical evidence
Studies have shown that Ang-2 increases inflammation and vascular leakage, and facilitates the effects of VEGF.2,4-7 Review the evidence below.

Hear from the experts:
Vascular leakage and neovascularisation
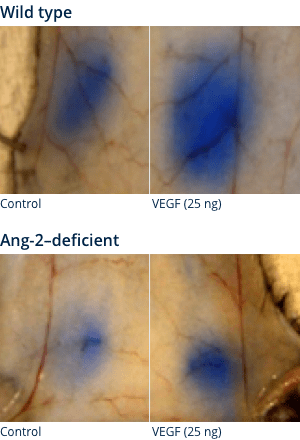
MOUSE SKIN MODEL MILES ASSAY
Ang-2 may act as a facilitator of VEGF-induced vascular leakage5
Visualisation of vascular leakage
Quantification of vascular leakage

Wild type
Ang-2–deficient mice
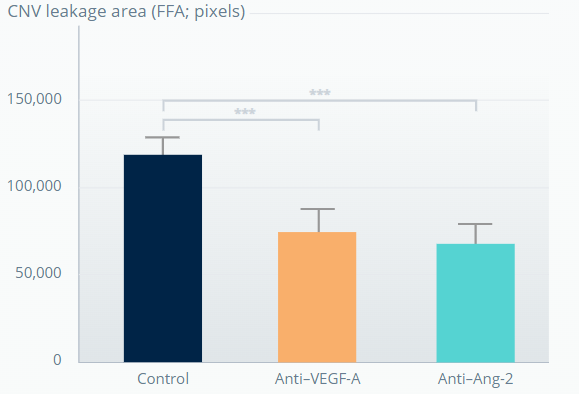
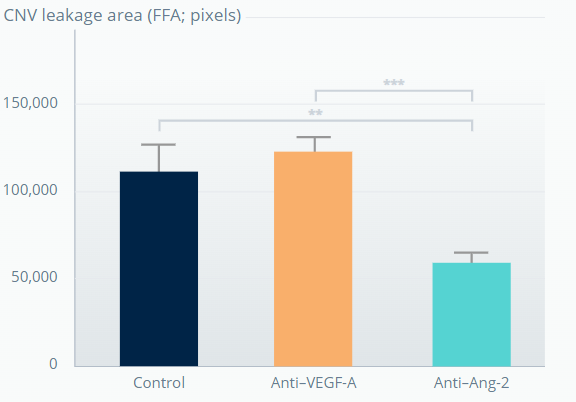
SPONTANEOUS CNV MOUSE MODEL†
Ang-2 inhibition promotes sustained reduction of vascular leakage from CNV lesions6
1 week after last antibody dose
5 weeks after last antibody dose
Wild type
Ang-2–deficient mice
**p<0.01; ***p<0.001. Error bars represent means±SD.
†The experiment involved 7-week-old JR5558 mice (5–10 mice per group). Figures adapted from Canonica J, et al. Poster [no. 628] presented at EURETINA, October 2020, Virtual Meeting. .
The role of pericytes in retinal vascular stability
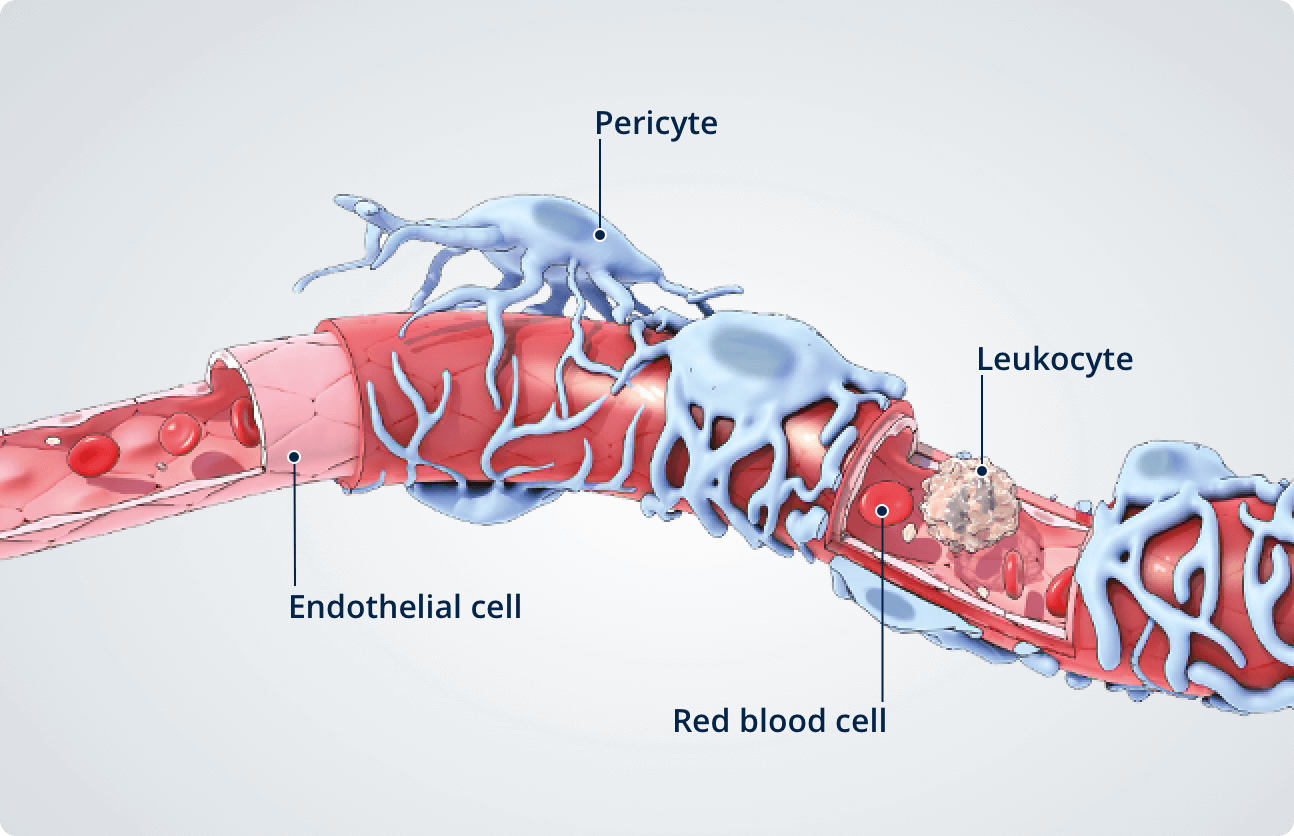
Healthy retina7,8
In the healthy retina, pericytes line the outside of endothelial cells and are critical for retinal vessel formation, stability, and generation and maintenance of the blood retinal barrier (BRB).
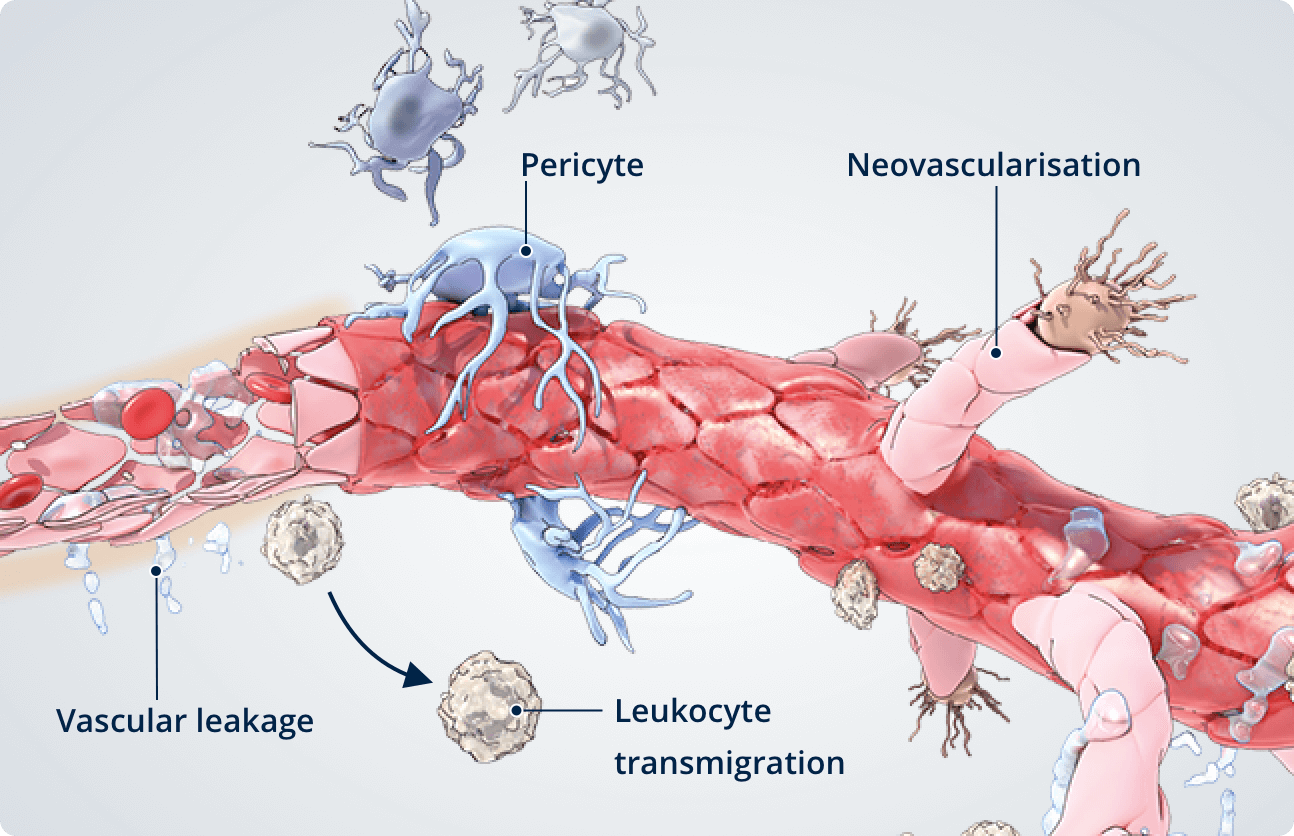
Diseased retina7,8
In the diseased retina, a deficiency of pericytes is associated with reduced barrier function and vascular instability.
- Pericyte dropout or apoptosis is a common feature of diabetic retinopathy.
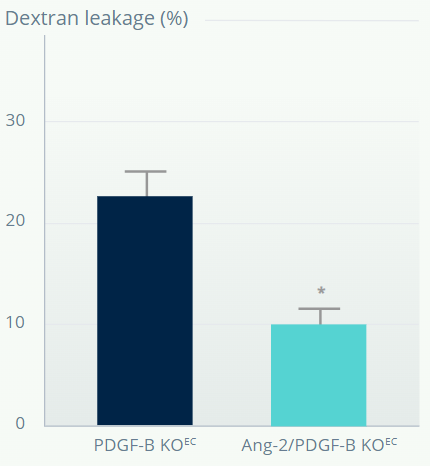
PDGF-B–DEFICIENT MOUSE MODEL†
Ang-2 inhibition reduces vascular leakage in retinas with depleted pericytes and BRB impairment7
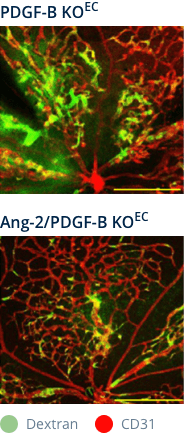
Ang-2 genetic deletion in
PDGF-B–deficient mice
IVT anti–Ang-2 in
PDGF-B–deficient mice
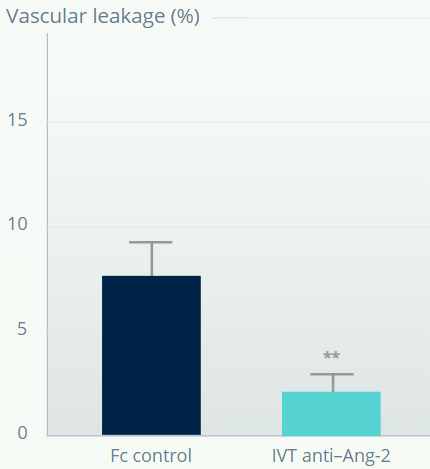
*p<0.05 versus pericyte depleted retina. **p<0.01 versus Fc control. Error bars represent means±SD.
†Mouse model of pericyte depletion in postnatal retina with PDGF-B deficiency.
Figures adapted from Park DY, et al. Nat Commun. 2017;16:15296.
Vascular leakage is reduced in retinas with depleted pericytes following Ang-2 inhibition.
Inflammation
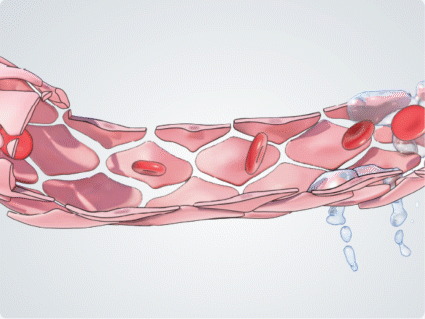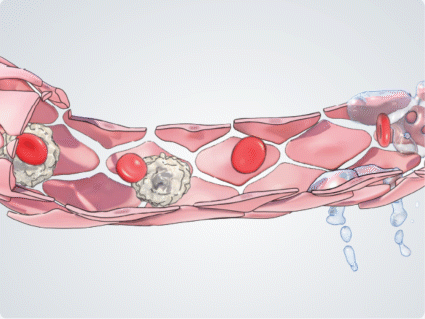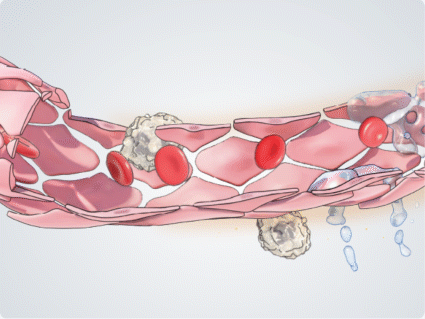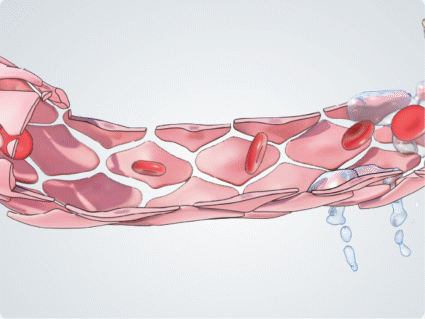
During inflammation, cytokines such as TNF-α induce the expression of adhesion molecules on the endothelial cell surface, mediating leukocyte tethering and rolling. Ang-2 acts as an amplifier to activate and sensitise vascular endothelial cells to inflammatory cytokines, regulating the transition from leukocyte rolling to firm adhesion and facilitating leukocyte migration across the vascular endothelium, into tissues such as the retina.9,10
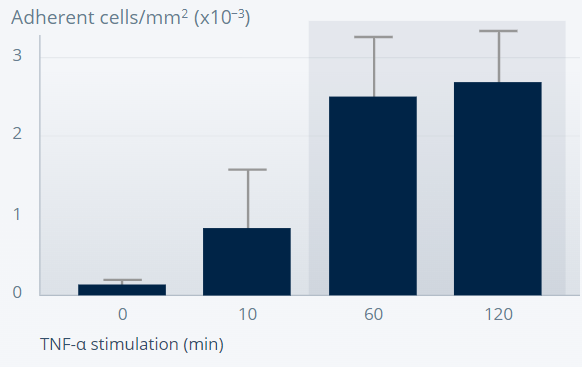
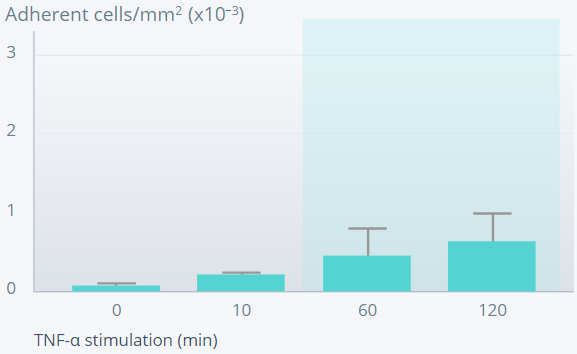
MOUSE DORSAL SKINFOLD CHAMBER MODEL
Ang-2 promotes leukocyte adhesion and transmigration into inflamed tissues.10
Wild type
Ang-2–deficient
Error bars represent means±SD.
Figures reprinted from Nature Medicine, 12(2), Fiedler U, et al., Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation, 235–9, Copyright (2006), with permission from Springer Nature.
Leukocyte adhesion is defective in Ang-2–deficient mice, suggesting an important role of Ang-2 in the inflammatory response.
SPONTANEOUS CNV MOUSE MODEL†
Ang-2 inhibition causes sustained prevention of subretinal macrophage infiltration from CNV lesions11
1 week after last antibody dose
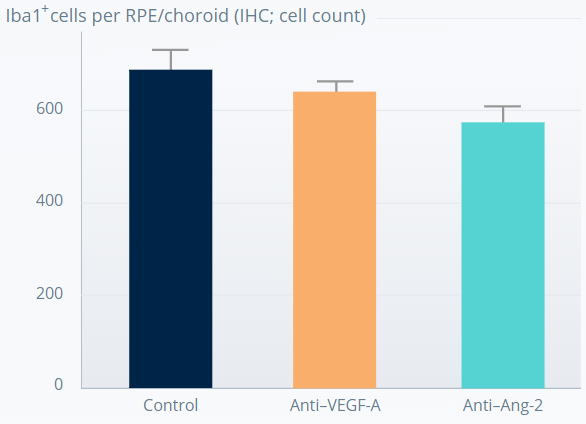
5 weeks after last antibody dose
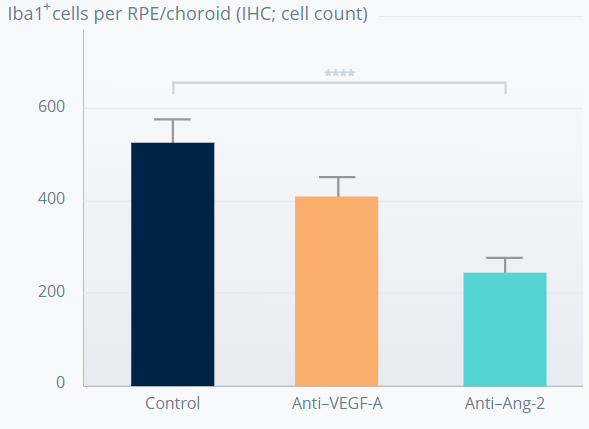
****p<0.0001. Error bars represent means±SD.
†The experiment involved 7-week old JR5558 mice (6–18 mice per group). Figures adapted from Canonica J, et al. Poster presented at ARVO, May 2021, Virtual Meeting.
The anti-inflammatory effect appears to be driven by Ang-2 inhibition.
References
- Saharinen P, et al. Nat Rev Drug Discov. 2017;16:635–61
- Joussen AM, et al. Eye. 2021;35:1305–16
- Regula JT, et al. EMBO Mol Med. 2019;11:e10666
- Regula JT, et al. EMBO Mol Med. 2016;8(11):1265–88
- Benest AV, et al. PLoS One. 2013;8:e70459
- Canonica J, et al. Poster [no. 628] presented at EURETINA, October 2020, Virtual Meeting
- Park DY, et al. Nat Commun. 2017;16:15296
- Santos GSP, et al. Eye. 2018;32:483–6
- Augustin HG, et al. Nat Rev Mol Cell Biol. 2009;10:165–77
- Fiedler U, et al. Nat Med. 2006;12:235–9
- Canonica J, et al. Poster presented at ARVO, May 2021, Virtual Meeting
