
Disease
PNH Prognosis
PNH was first described as a distinct clinical entity in 1882; however the link to dysregulated complement regulatory proteins was only established in 1983. If left untreated, PNH can be fatal, with roughly 35% of patients dying after five years and a median survival of 10 years after diagnosis.1-5
Supportive treatments to alleviate anaemia and manage haemolysis were standard of care for people with PNH for a long time, including blood transfusions, corticosteroids and immunosuppressive therapy. Bone marrow transplantation, the only curative option for people with PNH, comes with considerable safety risks and is only applicable for selected patients at present.6
The introduction of the C5 inhibitor class in 2007 has dramatically changed the treatment landscape for people with PNH. C5 inhibitors reduce haemolysis and thromboembolic complications as well as prolong survival in people with PNH, leading to over 90% 5-year survival rate.7,8
Over the years, limitations of current therapies have become apparent. Second-generation complement inhibitors focus on optimising the therapy for people with PNH by extended half-lives, different routes of administration or targeting the proximal complement activation.9-11
PNH Epidemiology

PNH Symptoms and complications
Ongoing haemolysis leads to the release of free haemoglobin into circulation, contributing to the pathophysiology of PNH and ultimately to many signs and symptoms of the disease.13
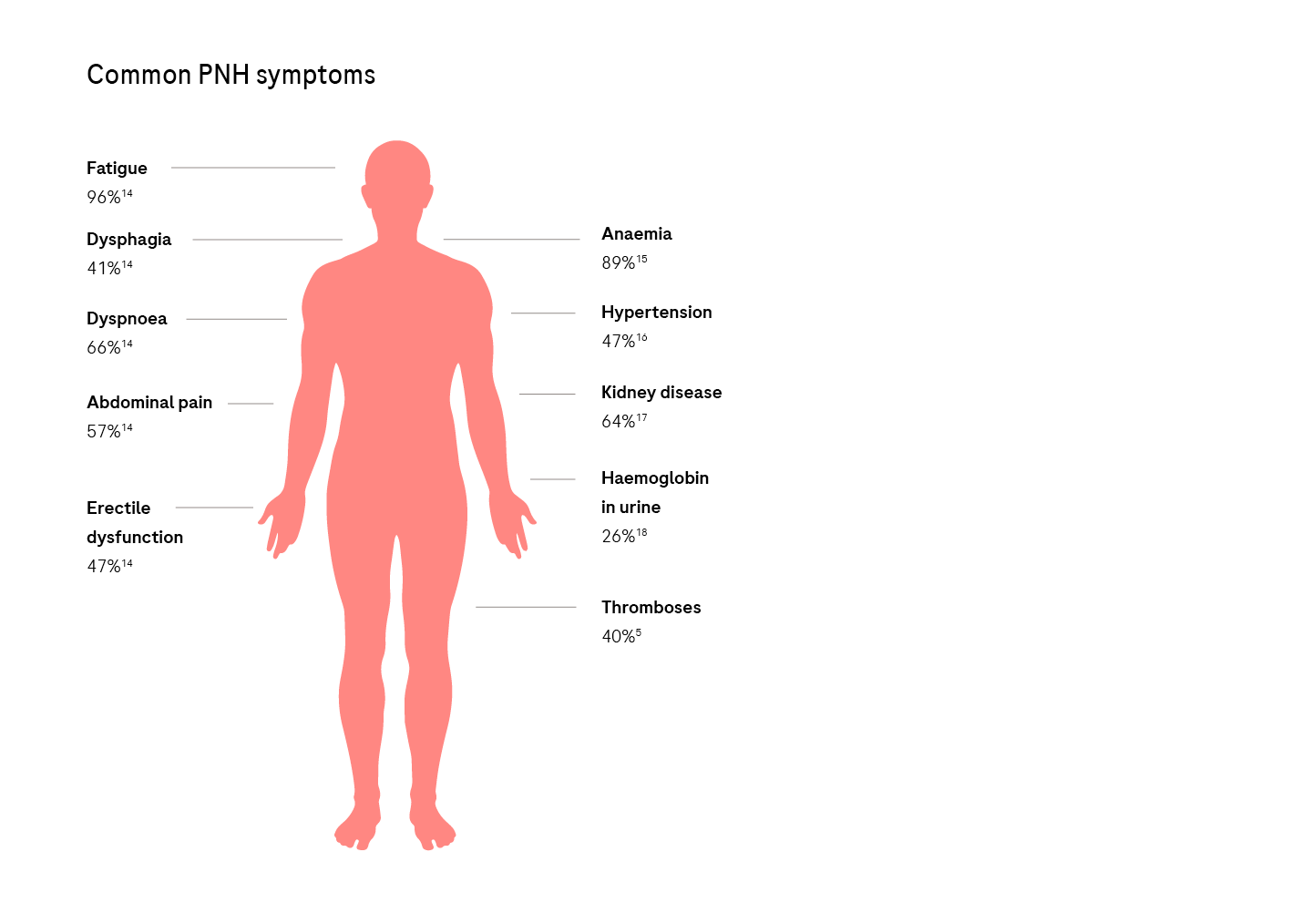
Clinical manifestations include anaemia, fatigue and haemoglobinuria, as well as smooth muscle dystonia reflected by abdominal and back pain, dysphagia and erectile dysfunction.2
Thrombosis is the most significant cause of morbidity and mortality in untreated PNH, attributed to almost 50% of PNH-related deaths. Additionally, people with a large PNH clone size are at greater risk for thrombosis.2,19
Learn more about the PNH Pathophysiology
and explore other PNH resources
References:
- Crosby WH. Paroxysmal nocturnal hemoglobinuria; a classic description by Paul Strübling in 1882, and a bibliography of the disease. Blood. 1951; 6 (3): 270–284.
- Hill A, DeZern AE, Kinoshita T et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017; 3: 17028. doi:10.1038/nrdp.2017.28
- Socié G, Mary JY, de Gramont A et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet. 1996; 348 (9027): 573–577. doi:10.1016/s0140-6736(95)12360-1
- de Latour RP, Mary JY, Salanoubat C et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008; 112 (8): 3099–3106. doi:10.1182/blood-2008-01-133918
- Hillmen P, Lewis SM, Bessler M et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995; 333 (19): 1253–1258. doi:10.1056/NEJM199511093331904
- Bektas M, Copley-Merriman C, Khan S et al. Paroxysmal nocturnal hemoglobinuria: current treatments and unmet needs. J Manag Care Spec Pharm. 2020; 26 (Suppl 12b): S14–S20. doi:10.18553/jmcp.2020.26.12-b.s14
- Risitano AM, Marotta S, Ricci P et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019; 10: 1157. doi:10.3389/fimmu.2019.01157
- Hillmen P, Muus P, Röth A et al. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013; 162 (1): 62–73. doi:10.1111/bjh.12347
- Kulasekararaj AG, Lazana I. Paroxysmal nocturnal hemoglobinuria: Where are we going. Am J Hematol. 2023; 98 (Suppl 4): S33–S43. doi:10.1002/ajh.26882
- Peffault de Latour R, Hosokawa K, Risitano AM. Hemolytic paroxysmal nocturnal hemoglobinuria: 20 years of medical progress. Semin Hematol. 2022; 59 (1): 38–46. doi:10.1053/j.seminhematol.2022.01.001
- Kulasekararaj AG, Risitano AM, Maciejewski JP et al. Phase 2 study of danicopan in patients with paroxysmal nocturnal hemoglobinuria with an inadequate response to eculizumab. Blood. 2021; 138 (20): 1928–1938. doi:10.1182/blood.2021011388
- Gulbis B, Eleftheriou A, Angastiniotis M et al. Epidemiology of rare anaemias in Europe. Adv Exp Med Biol. 2010; 686: 375–396. doi:10.1007/978-90-481-9485-8_22
- Rapido F. The potential adverse effects of haemolysis. Blood Transfus. 2017; 15 (3): 218–221. doi:10.2450/2017.0311-16
- Meyers G, Weitz I, Lamy T et al. Disease-related symptoms reported across a broad population of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007; 110 (11): 3683. https://doi.org/10.1182/blood.V110.11.3683.3683.
- Nishimura JI, Kanakura Y, Ware RE et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore). 2004; 83 (3): 193–207. doi:10.1097/01.md.0000126763.68170.46
- Hill A, Rother RP, Wang X et al. Effect of eculizumab on haemolysis-associated nitric oxide depletion, dyspnoea, and measures of pulmonary hypertension in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2010; 149 (3): 414–425. doi:10.1111/j.1365-2141.2010.08096.x
- Hillmen P, Elebute M, Kelly R et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010; 85 (8): 553–559. doi:10.1002/ajh.21757
- Parker C, Omine M, Richards S et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005; 106 (12): 3699–3709. doi:10.1182/blood-2005-04-1717
- Schrezenmeier H, Muus P, Socié G et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica. 2014; 99 (5): 922–929. doi:10.3324/haematol.2013.093161
