
Screening, diagnosis, and early detection
Improved surveillance and diagnostics open the possibility of earlier detection and curative therapies for HCC1
More than 50% of patients are diagnosed late or incidentally, removing the chance for curative therapies2,3
Delayed hepatocellular carcinoma (HCC) diagnosis is due to lack of symptoms, lack of awareness and screening, and limited sensitivity or access to diagnostic approaches1,4 – dramatically worsening prognosis and limiting curative options.2,3
HCC the silent killer
HCC has few specific symptoms, especially in the early stages. Combined with a general lack of awareness, a wide range of etiologies, and paucity of screening programs, diagnosis in the early stages of disease is understandably complex.2,5
Signs and symptoms
Early stage disease is difficult to detect and diagnose, as symptoms are rare6,7 and conventional liver tests have poor sensitivity.1
In advanced disease, approximately half of patients are identified incidentally following development of abdominal pain, weight loss, or worsening liver dysfunction.2

Abdominal pain/enlarged abdomen

Unexplained weight loss

Easy bleeding
or bruising

Jaundice
Risk factors for developing HCC
The risk of developing HCC increases with older age, and is greater among males than females.8,9 In addition, HCC risk can vary depending on different etiologies of origin: viral or metabolic. There has been a recent shift of HCC causes towards metabolic liver diseases, which has significantly increased the potential patient population and the need for a stepwise approach to risk stratification models to optimize healthcare resources.10
Prevention of HCC risk factors
Hepatitis B (HBV) is a virus that induces chronic liver inflammation leading to an increased risk of developing HCC. HBV vaccination programs are a key HCC prevention strategy, with many countries implementing programmes in the 1980s. Antiviral treatment of HBV infection can also improve liver function.9
Chronic hepatitis C virus (HCV) infection is a firmly established risk factor for HCC. There is no vaccine available to prevent HCV infection. Currently, antiviral treatment (Direct Acting Antiviral: DAA) of HCV infection decreases the risk of HCC developing in these patients.9
The changing trends in HCC etiology from viral towards metabolic suggest that more effort needs to be focused on combating obesity and diabetes to decrease the incidence of NAFLD, with more effective strategies required to identify and control alcohol misuse.9

HCC surveillance is associated with improved early detection, use of curative treatment, and prolonged survival times11-15

Early diagnosis enables initiation of treatment or behavioural changes to prevent disease progression and improve survival1
Patients with risk factors for HCC should undergo surveillance and regular monitoring when curative treatments are feasible.5,11 Identification of tumors at an early stage is critical, as curative treatments exist and provide the best opportunity for a positive prognosis.16 While it is recognised that an estimated 90% of HCC cases are associated with liver cirrhosis, there is still a need for reliable and effective HCC risk prediction models based on routinely available patient factors.17,18
Currently, HCC diagnosis is often delayed due to lack of symptoms and awareness, with advanced stage associated with significantly worse prognosis2,3 as curative treatment becomes unavailable and systemic management options are limited.2,16 International guidance for HCC recommends that surveillance in at-risk patients should consist of a program of measures, including standardized screening tests (including ultrasound screening every 6 months), recall procedures, and quality control procedures.19

Although recommended by several guidelines, HCC surveillance is limited and underutilised in real-world practice
Despite the guideline recommendations, real-life implementation of surveillance programs is far from optimal, with ≤50% of the high-risk population estimated to not receive routine screening, even in countries with surveillance programmes.20,21 Thus, over 60% of HCC cases are diagnosed at an intermediate or advanced stages11 – limiting patient eligibility for curative options, and reducing survival outcomes.2 In patients undergoing surveillance, HCC-related mortality decreases by over a third compared with those not screened for HCC.2
This can contribute to the many barriers that HCPs and patients face:
- • Screening activities are de-prioritised due to time, capacity, and healthcare funding constraints22
- • Some patients struggle to attend appointments due to scheduling and living distances from treatment centers23
- • Low awareness of early diagnosis and need to screen high-risk patients13
- • Stigmatisation of patients may result in non-attendance at scheduled hospital24-26
- • Recommended screening/diagnostic imaging is not reimbursed in all countries4
- • As HCC is rarely identified in Primary care, patients may experience delays in referrals22
Less than 50% of patients are identified with early disease11
Early identification of people at risk of developing HCC should rely on multimodal information: clinical, imaging and molecular
Currently, screening biomarkers for HCC are lacking and rarely implemented in clinical practice.10 The sensitivity of AFP testing, even when combined with ultrasound screening, remains suboptimal in detecting early-stage HCC.27
Early detection of HCC is essential to improve patient outcomes, and current guidelines recommend surveillance programs to screen at-risk patients, including ultrasound scans every 6 months with or without alpha-fetoprotein (AFP) testing.28

Opportunity for improved HCC detection via GAAD or GALAD algorithms
Serum biomarkers, such as AFP, protein induced by vitamin K absence-II (PIVKA-II) and Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), have been proposed to improve the detection of HCC28 but do not provide adequate specificity or sensitivity alone and inclusion in guidelines has been inconsistent.29,31 Both the GAAD and GALAD algorithms* are in vitro diagnostic multivariate index assays combining patient data points to provide a semi-quantitative result.31
The GAAD algorithm combines gender (sex) and age with two biomarkers (AFP and PIVKA-II), while the GALAD algorithm combines gender and age with three-serum biomarkers (AFP-L3, AFP and PIVKA-II).31 Both GAAD and GALAD algorithmic scores demonstrate equivalent clinical performance for HCC surveillance in prospective, multicenter, case-control studies,28 irrespective of etiology and disease stage.31
GAAD and GALAD algorithms demonstrate good and similar performance for the clinical detection of early-stage and all-stage HCC.28,31-34

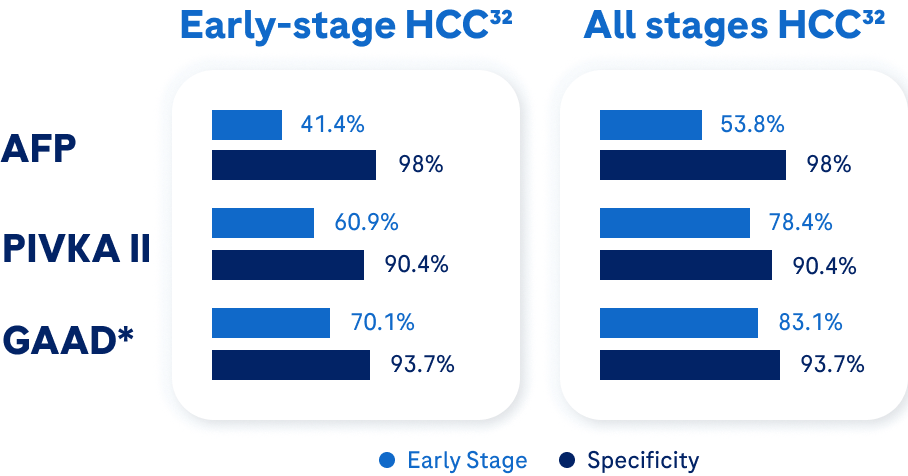
The GAAD algorithm* offers 92% specificity† and 73% sensitivity† for detection of early-stage HCC – superior to individual biomarkers alone.32
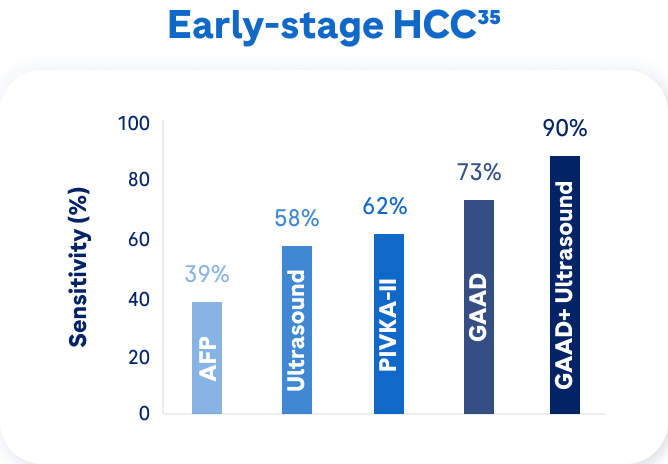
When combined with ultrasound, the GAAD algorithm* offers a sensitivity of approximately 90% in early-stage HCC,35 demonstrating its utility as a complementary tool in early-stage HCC detection.33
GAAD* also demonstrates cost-effectiveness (gain of 0.01 QALYs) compared with US+AFP for HCC, and could lead to better outcomes for HCC patients and save healthcare resources.36

*based on use with Elecsys® assays‡
† Sensitivity is the probability of a positive test result truly being positive; specificity is the probability of a negative test result truly being negative
‡ Assay results from other manufacturers have not been validated for the use of the Elecsys® GAAD test. The Elecsys® GAAD test should not be used without an independent clinical/radiological evaluation for the diagnosis of Hepatocellular Carcinoma (HCC). For each Elecsys® GAAD determination the measurement of the Elecsys® AFP and Elecsys® PIVKA-II assays must be determined from the same sample and measurement performed on the same analyzer type (either on cobas e 402, cobas e 411, cobas e 601, cobas e 602, or cobas e 801 analyzers).
References
- Ginès P, et al. Hepatology. 2022;75(1):219–22.
- Llovet JM, et al. Nat Rev Dis Primers. 2021;7(1):6.
- Yang JD, et al. Nat Rev Gastroenterol Hepatol 2019;16(10):589–604.
- Kardashian A, et al. Hepatology. 2023;77:1382–1403.
- Sharma A, et al. Chronic Liver Disease. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554597/
- Vogel A, et al. Ann Oncol. 2021;32(6):801–5.
- Reig M, et al. Journal of Hepatology 2022;76:681–93.
- Rumgay H, et al. J. Hepatol. 2022;77:1598–1606.
- McGlynn KA, et al. Hepatology 2021;73(suppl1):4–13.
- Lee Y-T, et al. Hepatology 2023;78(1):31–62.
- Del Poggio P, et al. World J Gastroenterol 2021;27(37):6180–90.
- Singal AG, et al. PLoS Med 2014;11:e1001624.
- Singal AG, et al. Am J Med 2017; 130:1099–106.
- Cadier B, et al. Hepatology 2017;65:1237–48.
- Mourad A, et al. Hepatology 2014;59:1471–81.
- Zhu XD, et al. Genes & Diseases 2020;7:359–69.
- Liu Y, et al. Front. Public Health 2022;10:955287.
- Fattovich G, et al. Gastroenterology 2004;127:S35–S50.
- NCCN Clinical Practice Guidelines in Oncology. Hepatocellular Carcinoma V1.2023.
- Xu K, et al. Ann Glob Health 2017;83:281–92.
- Singal AG. Clin Gastroenterol Hepatol. 2015;13(12):2140–51.
- Beal EW. et al. Provider- and system-level barriers to surveillance for hepatocellular carcinoma among patients with chronic liver disease. ASCO GI 2022 (Abstract 404).
- Farvardin S, et al. Hepatology. 2017;65(3):875–84.
- Carol M, et al. PLoS ONE 2022;17(4):e0265153.
- Schomerus G, et al. J Hepatol 2022;77:516–24.
- Vaughn-Sandler V, et al. Dig Dis Sci 2014;59(3):681–6.
- Singal AG, et al. Hepatology Communications. 2022;6:2925–36.
- Hou J, Berg T, Vogel A, et al. GAAD and GALAD algorithmic scores demonstrate equivalent clinical performance for hepatocellular carcinoma (HCC) surveillance in prospective, multicenter, case-control studies. Presented at EuroMedLab 2023: May 21-25, Rome, Italy. Poster P2089.
- Piratvisuth T, et al. Hepatol Commun. 2022;6(4):679–91.
- Parikh ND, et al. Cancer Epidemiol Biomarkers Prev. 2020;29(12):2495–2503.
- Chan HLY, et al. J Hepatol. 2022;77:S937.
- Teerha Piratvisuth ET AL. Hepatol Commun. 2023 Nov 8;7(11):e0317. doi: 10.1097/HC9.0000000000000317. eCollection 2023 Nov 1. Available from: https://pubmed.ncbi.nlm.nih.gov/37938100/
- Huang CF; Kroeniger K; Wang CW, et al. Clinical Performance of GAAD and GALAD Algorithmic Scores for Hepatocellular Carcinoma Surveillance in Patients with Chronic Hepatitis. Presented at International Liver Cancer Association (ILCA) 2023: September 7–9, Amsterdam. Poster P-72.
- Chan HLY, Vogel A, Berg T, et al. Performance Evaluation of the Elecsys® GAAD Assay for the Detection of Hepatocellular Carcinoma Across Different Disease Stages and Etiologies. Presented at ISHVLD Global Hepatitis Summit 2021: June 18–20, Taipei, Taiwan [Virtual].
- Huang CF, Sharna A, Yu ML. The clinical utility of Elecsys GAAD score in the diagnosis of hepatocellular carcinoma. Presented at Asian Pacific Association for the Study of the Liver (APASL) 2022: June 23-25, Taipei, Taiwan. Poster P161.
- Garay OU, Ambühl LE, Bird T, et al. Cost-utility analysis of the Elecsys GAAD algorithm versus ultrasound plus α-fetoprotein for HCC surveillance in patients with compensated liver cirrhosis in the United Kingdom. Presented at ISPOR Europe 2022: November 6-9, Vienna. Poster EE37.







