
About oncogenetic drivers
ALK is a therapeutic target in NSCLC, with
gene fusions occurring in ~5%
of patients5-7
Patients with ALK+ NSCLC require an effective treatment with both systemic and central nervous system (CNS) activity3–6
The most common sites of metastasis for patients with all types of stage IV lung cancer8
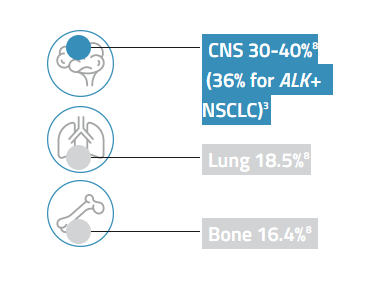
Despite advances in therapy for ALK+ NSCLC, additional treatment options are needed to improve clinical outcomes
High-quality testing such as IHC, FISH and NGS are needed to identify patients with actionable ALK gene fusions9
Testing for ALK+ rearrangement should be systematically carried out in advanced NSCLC10
- ALK testing involves the detection of ALK gene rearrangements or overexpression of ALK proteins.11
- FISH, IHC and RT-PCR are currently used in routine clinical practice.
- NGS offers more comprehensive detection of ALK fusions and other oncogenic driver alterations.

NGS is increasingly being used and is a sensitive and accurate test to identify most ALK gene fusions with a single test9,13–19
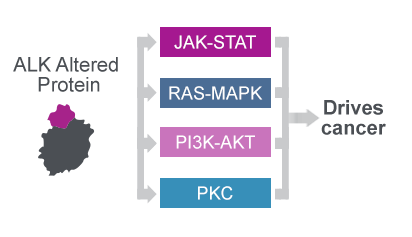
The ALK protein plays an important role in healthy tissue and if altered by fusion events or mutations drives cancer through aberrant signalling26
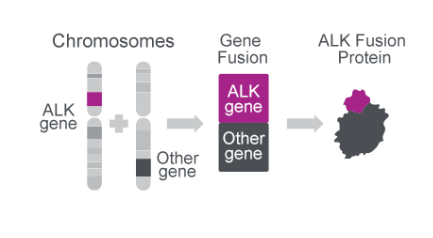
ALK gene fusions create oncogenic proteins
- The ALK gene can combine with multiple other gene partners to create an oncogenic fusion protein26
- So far, ~30 different fusions have been detected26
The oncogenic ALK protein drives cancer through aberrant signalling26
- The oncogenic fusion or mutated ALK protein constitutively activates signalling cascades implicated in cell proliferation, survival and angiogenesis26
Footnotes:
CNS, central nervous system; FISH, fluorescence in situ hybridisation; IHC, immunohistochemistry; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; RT-PCR, reverse transcription polymerase chain reaction
1.Bergethon K, et al. J Clin Oncol 2012;30:863–870.
2. Dugay F, et al. Oncotarget 2017;8:53336–53351.
3. Patil T, et al. J Thorac Oncol 2018;13:1717–1726.
4. Gainor JF, et al. JCO Precis Oncol 2017. DOI: 10.1200/PO.17.00063.
5. Qiu Z et al. Sci Rep 2020;10:10387;
6. Gainor JF, Shaw AT. Oncologist 2013;18:865–875;
7. Genentech USA, Inc. ALECENSA Prescribing Information. 2021;
8. Oikawa A, et al. Oncol Lett 2012;3:629–634.
9. Bubendorf L, et al. Virchows Arch 2016;469:489–503.
10. Planchard D. Ann Oncol 2018;29:iv192–iv237.
11. International Association for the Study of Lung Cancer. IASLC Atlas of ALK and ROS1 testing in lung cancer. Available at: https://www.iaslc.org/research-education/publications-resources-guidelines/iaslc-atlas-alk-and-ros1-testing-lung-cancer (Accessed November 2020).
12. Rossi G, et al. Lung Cancer (Auckl) 2017:8:45–55.
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. V.6.2020, 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed November 2020).
14. Diaz L, Bardelli A. J Clin Oncol 2014;32:579–586.
15. Shan L, et al. PLoS One 2015;10:e0120422.
16. Cao B, et al. Onco Targets Ther 2016;31:131–138.
17. Zheng Z, et al. Nat Med 2014;20:1479–1484.
18. Drilon A, et al. Clin Cancer Res 2015;21:3631–3639.
19. Grada A, Weinbrecht K. J Invest Dermatol 2013;133:e11.
20. Birchmeier C, et al. Proc Natl Acad Sci U S A 1987;84: 9270–9274.
21. Rikova K, et al. Cell 2007;131:1190–1203.
22. Gainor J, Shaw A. Oncologist 2013;18:865–875.
26. Du. et al. Thoracic Cancer 2018; 9(4): 423-430
