
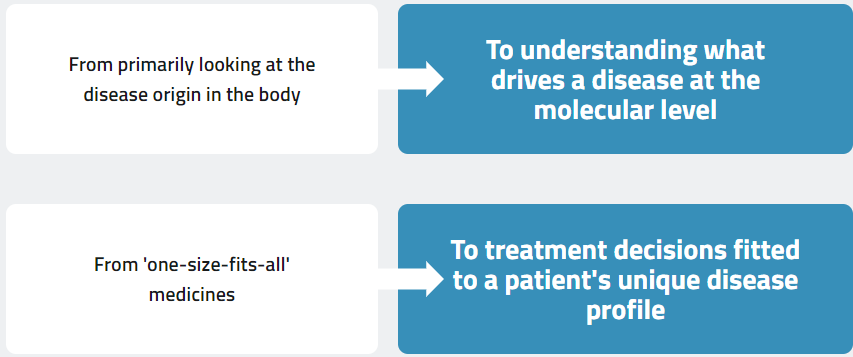
About oncogenetic drivers
Knowing a patient’s biomarker profile can inform treatment decisions and open up
new targeted therapy options1–3
High-quality next-generation sequencing (NGS) helps you see beyond the tumour origin to exactly which mutations are driving that cancer.4
What’s changing5,6
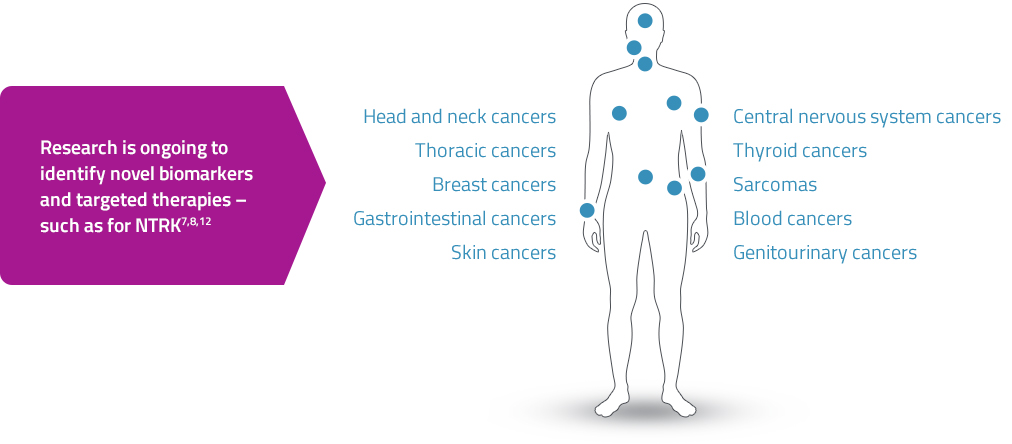
Targeted therapies are being studied to advance treatment options for patients with actionable biomarkers7,8
- Biomarkers such as ALK, BRAF, EGFR, HER2, KIT and ROS1 have changed the course of therapy in multiple cancer types9-11
- NTRK gene fusions are emerging as actionable biomarkers and oncogenic drivers7,8,12
High-quality NGS can help inform treatment decisions and open up new targeted therapy options for patients1,9,25,26
- NGS using targeted gene panels (~50 genes) can identify most actionable mutations and fusions
- Comprehensive Genomic Profiling (CGP) (>300 genes) also uses NGS to analyze the cancer genome and offers broad coverage of clinically relevant alterations, potentially expanding patients' treatment options4,26–30
- NTRK, ROS1, ALK gene fusions as well as RET gene alterations can be accurately detected with Comprehensive Genomic Profiling (CGP)4,26–30
Precision medicine expands treatment options and approaches for patients, including traditional cancer therapies and emerging targeted therapies, with the aim of achieving the best possible outcome for the patient5
Footnotes:
CNS, central nervous system; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer.
1. Rozenblum AB, et al. J Thorac Oncol 2017;12:258–268.
2. Schwaederle M, Kurzrock R. Oncoscience 2015;2:779–780.
3. Mansinho A, et al. Expert Rev Anticancer Ther 2017;17:563–565.
4. Frampton GM, et al. Nat Biotechnol 2013;31:1023–1031.
5. Bode AM, Dong Z. NPJ Precis Oncol 2018;2:1.
6. Roche.com. What is Personalised Healthcare all about? Available from: https://www.roche.com/about/priorities/personalised_healthcare/what_is_phc.htm (Accessed November 2020).
7. Vaishnavi A, Le AT, Doebele RC. Cancer Discov 2015;5:25–34.
8. Amatu A, Sartore-Bianchi A, Siena S. ESMO Open 2016;1:e000023.
9. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines).
Non-Small Cell Lung Cancer. V.6.2020, 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed November 2020).
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. V.6.2020, September 8 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed November 2020).
11. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. V.1.2021, October 14 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (Accessed November 2020).
12. Lange AM, Lo HW. Cancers (Basel) 2018;10.
13. Baumgart M, Pandya K. Exp Rev Precis Med Drug Dev 2016;1:25–36.
14. Leonetti A, et al. Cancer Treat Rev 2018;66:82–94.
15. European Society for Medical Oncology. BRAF in Colorectal Cancer: ESMO Biomarker Factsheet. Available at: https://oncologypro.esmo.org/Education-Library/Factsheets-on-Biomarkers/BRAF-in-Colorectal-Cancer (Accessed November 2020).
16. Dearden S, et al. Ann Oncol 2013;24:2371–2376.
17. Tsao S, et al. J Thorac Oncol 2016;11:613–638.
18. European Society for Medical Oncology. HER2 in Breast Cancer: ESMO Biomarker Factsheet. Available at: https://oncologypro.esmo.org/Education-Library/Factsheets-on-Biomarkers/HER2-in-Breast-Cancer (Accessed November 2020).
19. Sakabe T, et al. Oncol Lett 2017;13:3703–3708.
20. Bergethon K, et al. J Clin Oncol 2012;30:863–870.
21. Dugay F, et al. Oncotarget 2017;8:53336–53351.
22. Davies K, Doebele RC. Clin Cancer Res 2013;19:4040–4045.
23. Lin J, Shaw A. J Thorac Oncol 2017;12:1611–1625.
24. Okamura R, et al. JCO Precis Oncol 2018;2018.
25. Gagan J, Van Allen EM. Genome Med 2015;7:80.
26. Suh JH, et al. Oncologist 2016;21:684–691.
27. Drilon A, et al. Clin Cancer Res 2015;21:3631–3639.
28. Rankin A, et al. Oncologist 2016;21:1306–1314.
29. Ross JS, et al. Cancer 2016;122:2654–2662.
30. Hirshfield KM, et al. Oncologist 2016;21:1315–1325.
