
About oncogenetic drivers
RET gene alterations occur in various
tumour types and are a therapeutic target
in thyroid cancer and NSCLC.1,2,8,9
The prevalence of oncogenic RET gene alterations (fusions or mutations) varies according to the tumour type.1–7
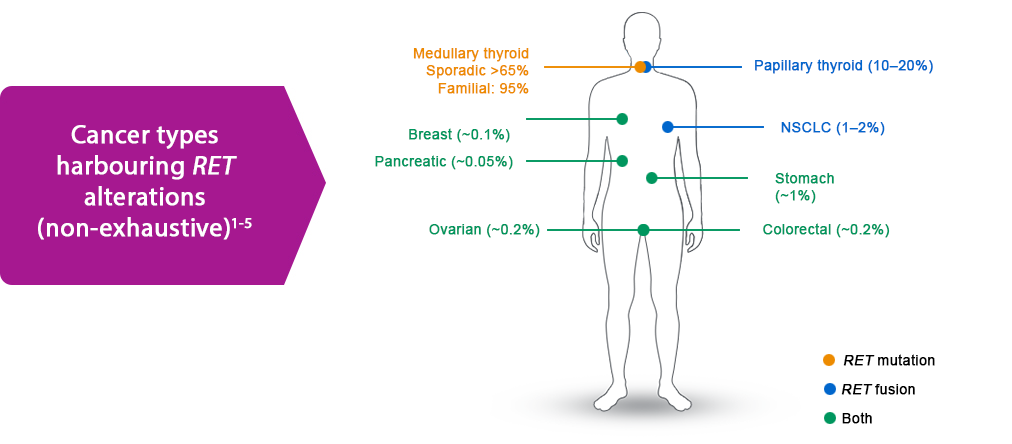
RET mutations are seen in approximately 70% of medullary thyroid cancers (MTC), including up to 95% of patients with familial MTC and >65% of patients with sporadic MTC.6,7
RET fusions are seen in 10–20% of papillary thyroid cancer (PTC) and other thyroid cancers. They are also found in 1–2% of NSCLC and in a variety of solid tumour at lower frequencies.1-9
Testing for RET brings the positives of precision therapy to patients with RET-altered cancers
ESMO recommends routine molecular testing for RET alterations in medullary thyroid cancer (MTC) and in non- MTC, NSCLC and other solid tumours by NGS or PCR.1,2
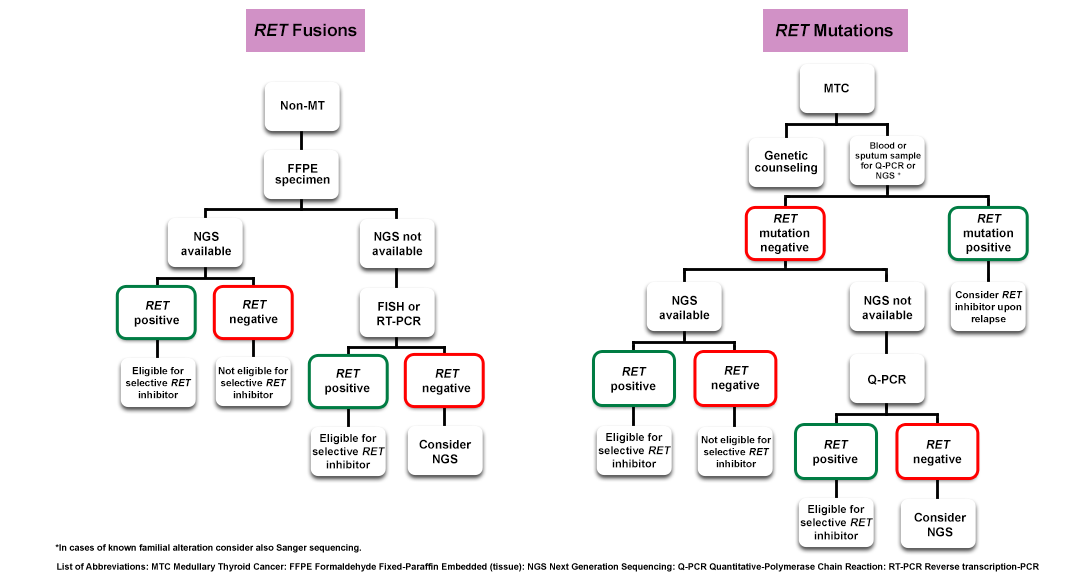
Testing for RET+ rearrangement should be systematically carried out in advanced NSCLC10
- RET testing involves the detection of RET gene rearrangements.11
- FISH and RT-PCR are currently used in routine clinical practice with NGS as an emerging technology

NGS is increasingly being used and is a sensitive and accurate test to identify most RET gene fusions with a single test9,13–19
FISH has been the standard approach to detecting RET gene rearrangements10
RT-PCR has good sensitivity and specificity; however, its use may be limited by the presence of numerous RET fusion partners, both identified and still unknown, and difficulties in obtaining good-quality RNA12
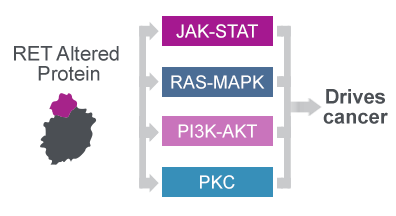
The RET protein plays an important role in healthy tissue and if altered by fusion events or mutations drives cancer through aberrant signalling27
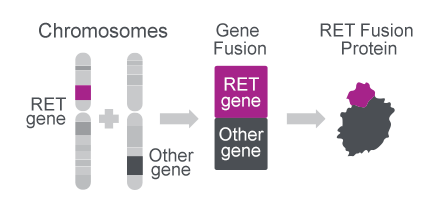
RET gene fusions create oncogenic proteins27
- The RET gene can combine with multiple other gene partners to create an oncogenic fusion protein27
- So far, more than 35 different RET fusion genes have been reported27
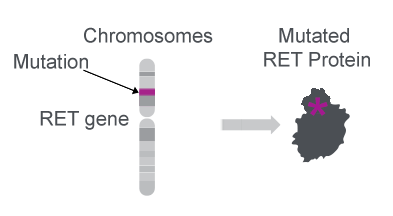
RET gene mutations create an oncogenic protein27
- The RET gene can be mutated to form an oncogenic protein27
The oncogenic RET protein drives cancer through aberrant signalling27
- The oncogenic fusion or mutated RET protein constitutively activates signalling cascades implicated in cell proliferation, survival and angiogenesis27
Footnotes:
CNS, central nervous system; FISH, fluorescence in situ hybridisation; IHC, immunohistochemistry; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; RT-PCR, reverse transcription polymerase chain reaction.
1. Bergethon K, et al. J Clin Oncol 2012;30:863–870.
2. Dugay F, et al. Oncotarget 2017;8:53336–53351.
3. Patil T, et al. J Thorac Oncol 2018;13:1717–1726.
4. Gainor JF, et al. JCO Precis Oncol 2017. DOI: 10.1200/PO.17.00063.
5. Qiu Z et al. Sci Rep 2020;10:10387;
6. Gainor JF, Shaw AT. Oncologist 2013;18:865–875;
7. Genentech USA, Inc. ALECENSA Prescribing Information. 2021;
8. Oikawa A, et al. Oncol Lett 2012;3:629–634.
9. Bubendorf L, et al. Virchows Arch 2016;469:489–503.
10. Planchard D. Ann Oncol 2018;29:iv192–iv237.
11. International Association for the Study of Lung Cancer. IASLC Atlas of ALK and ROS1 testing in lung cancer. Available at: https://www.iaslc.org/research-education/publications-resources-guidelines/iaslc-atlas-alk-and-ros1-testing-lung-cancer (Accessed November 2020).
12. Rossi G, et al. Lung Cancer (Auckl) 2017:8:45–55.
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. V.6.2020, 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed November 2020).
14. Diaz L, Bardelli A. J Clin Oncol 2014;32:579–586.
15. Shan L, et al. PLoS One 2015;10:e0120422.
16. Cao B, et al. Onco Targets Ther 2016;31:131–138.
17. Zheng Z, et al. Nat Med 2014;20:1479–1484.
18. Drilon A, et al. Clin Cancer Res 2015;21:3631–3639.
19. Grada A, Weinbrecht K. J Invest Dermatol 2013;133:e11.
20. Birchmeier C, et al. Proc Natl Acad Sci U S A 1987;84: 9270–9274.
21. Rikova K, et al. Cell 2007;131:1190–1203.
22. Gainor J, Shaw A. Oncologist 2013;18:865–875.
27. Thein et al; 2021; Trends in Cancer 2021; 7 1074-1088
