
For the first time in its history, the 73rd Annual Meeting of the American Academy of Neurology (AAN) – the world’s premier neurology meeting – was held in a fully virtual format from April 17th to 22nd 2021
Welcome to AAN 2021

James Stevens, AAN President
The 2021 virtual AAN annual meeting was centred around a rich educational and scientific programme including over 90 expert-led educational courses, more than 2000 scientific abstracts and seven key plenary sessions featuring leaders in neurology.
AAN President James Stevens, Fort Wayne, USA, began this key plenary session with presentation of the President’s Award followed by the Presidential Lecture entitled: ‘Disruption: How to Pivot from Uncertainty to Success - the AAN Story.’ Dr Stevens proposed a focused five-pronged approach to navigating crises such as COVID-19.
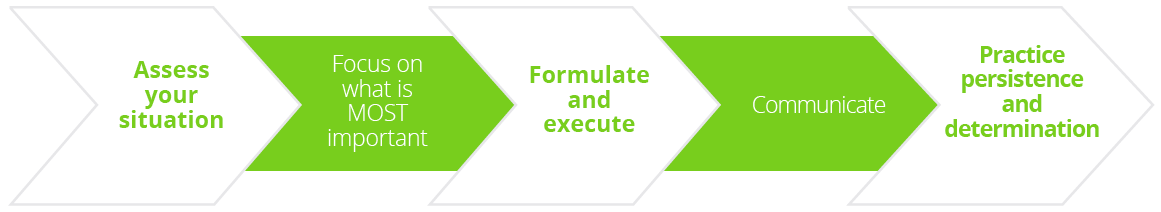
Five-pronged approach to navigating crises such as COVID-19.
“What a year it has been - unprecedented does not even begin to describe what we have all been through. We’ve faced uncertainty, exhaustion and challenge but we are emerging stronger, resilient and prepared to face our new future. ”
President James Stevens, Fort Wayne, USA

Orly Avitzur, AAN President-Elect
The 2021 virtual AAN annual meeting was centred around a rich educational and scientific programme including over 90 expert-led educational courses, more than 2000 scientific abstracts and seven key plenary sessions featuring leaders in neurology.
There then followed a brief welcome address by AAN President-Elect Orly Avitzur, Tarrytown, USA, who will be charged with leading the academy over the next two years. As we enter the recovery phase of the pandemic, Dr Avitzur outlined some exciting AAN programmes for the future including an increasing focus on hybrid educational and scientific meetings, studies to support telemedicine and an expanded emphasis on well-being.
NEUROMUSCULAR AND MOVEMENT DISORDERS
Update on clinical trials in Huntington’s disease
Tiago Mestre, Ottawa, Canada, provided an update on clinical trials in Huntington’s disease (HD). Although chorea currently remains the only symptom with an approved therapeutic indication (for tetrabenazine and deutetrabenazine), the pipeline of potential symptomatic treatments is broad - targeting key symptoms of HD such as motor impairment and chorea, as well as behavioural, cognitive and functional disease effects. Multiple target and disease-specific drug programmes are also in active clinical development pursuing the holy grail of disease modification in HD.
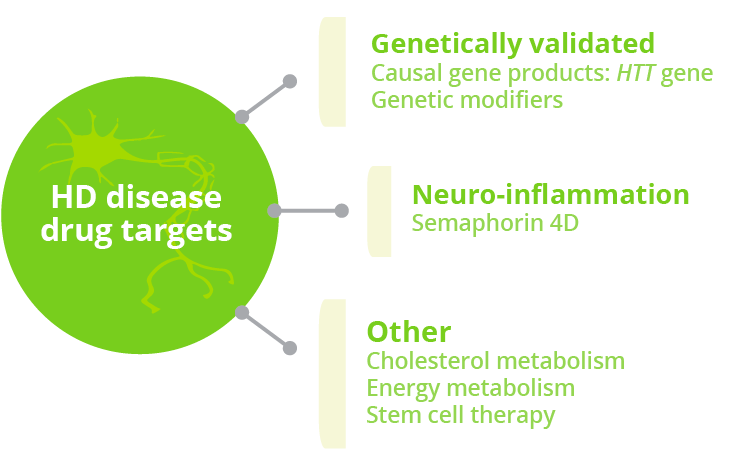
Huntington’s disease drug targets
Although the first phase III study of a gene therapy candidate for HD - the GENERATION HD1 trial (NCT03761849) of the non-allele specific antisense oligonucleotide (ASO) tominersen - had to be halted early due to concerns about the risk-benefit profile, other Phase I/II studies in the field remain ongoing. These include the PRECISION HD1 (NCT03225833) and HD2 (NCT03225846) studies involving allele-specific ASOs and the CT-AMT-130-01 trial (NCT04120493) of the non-allele specific micro RNA AMT-130-AAV5.
“Ongoing trials on motor, cognitive and behavioural features are relevant [in Huntington’s disease] even in a future scenario of an effective disease-modifying therapy.”
Tiago Mestre, Ottawa, Canada
Observational evidence in spinal muscular atrophy (SMA)
Maryam Oskoui, Montreal, Canada, showcased real-world motor outcomes data for patients with spinal muscular atrophy (SMA) included in the Canadian Neuromuscular Disease Registry (CDNR). Type I SMA patients initiated on nusinersen therapy at <6 months showed more dramatic improvements in CHOP-INTEND motor outcomes compared with those who started therapy at an older age. In the nusinersen-treated type I cohort, 7% (n=3) of patients ultimately switched to risdiplam and 26% (n=7) underwent gene therapy with onasemnogene abeparvovec. For later-onset patients with SMA, median age at therapy initiation was 9.0 years with the majority (80%, n=50) of patients receiving nusinersen, 5% (n=2) zolgensma and 15% (n=9) risdiplam. Dr Oskoui pointed out that older type II-IV SMA patients treated with nusinersen showed broad stability in their motor function compared with the decline that would be expected based on disease natural history.
Closing Remarks

Hailing this year’s annual meeting as ‘ANN excellence delivered unconventionally’ outgoing President Dr Stevens closed the congress by handing the reins of leadership to the new ANN President Dr Avitzur with the passing of the gavel. Dr Avitzur expressed her hope for an optimistic future with next year’s AAN meeting, due to take place in Seattle, Washington, between the 2nd and 8th April 2022, providing the opportunity for members to come back together again in person.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.