Welcome to ESMO 2021
Days 1 and 2 at the ESMO Congress 2021 saw some potentially practice-changing data presented in the fields of cervical and ovarian cancer, including the first-line use of pembrolizumab for advanced cervical cancer and olaparib rechallenge in ovarian cancer.
Cervical Cancer
First-line pembrolizumab ‘game changer’ in persistent, recurrent or metastatic cervical cancer
Interim analysis of phase 3 KEYNOTE-826 (NCT03635567) data suggests that adding the PD-1 inhibitor pembrolizumab to platinum-based chemotherapy and bevacizumab (where indicated) significantly delays disease progression and prolongs survival, relative to placebo, in people with persistent, recurrent, or metastatic cervical cancer.
“Overall, data from KEYNOTE-826 suggest that pembrolizumab plus chemotherapy with or without bevacizumab may be a new first-line standard of care for women with persistent, recurrent, or metastatic cervical cancer.”
Nicoletta Columbo, Milan, Italy
Speaking during Saturday’s Presidential Symposium, Nicoletta Columbo, from the European Institute of Oncology IRCCS in Milan, Italy, reported that, in the intention-to-treat population, the risk of disease progression or death was a significant 35% lower among the 308 women randomly assigned to receive pembrolizumab 200 mg every 3 weeks for up to 35 cycles than among the 309 given placebo. All participants also received concurrent paclitaxel and the investigator’s choice of cisplatin or carboplatin, and 63% received bevacizumab.
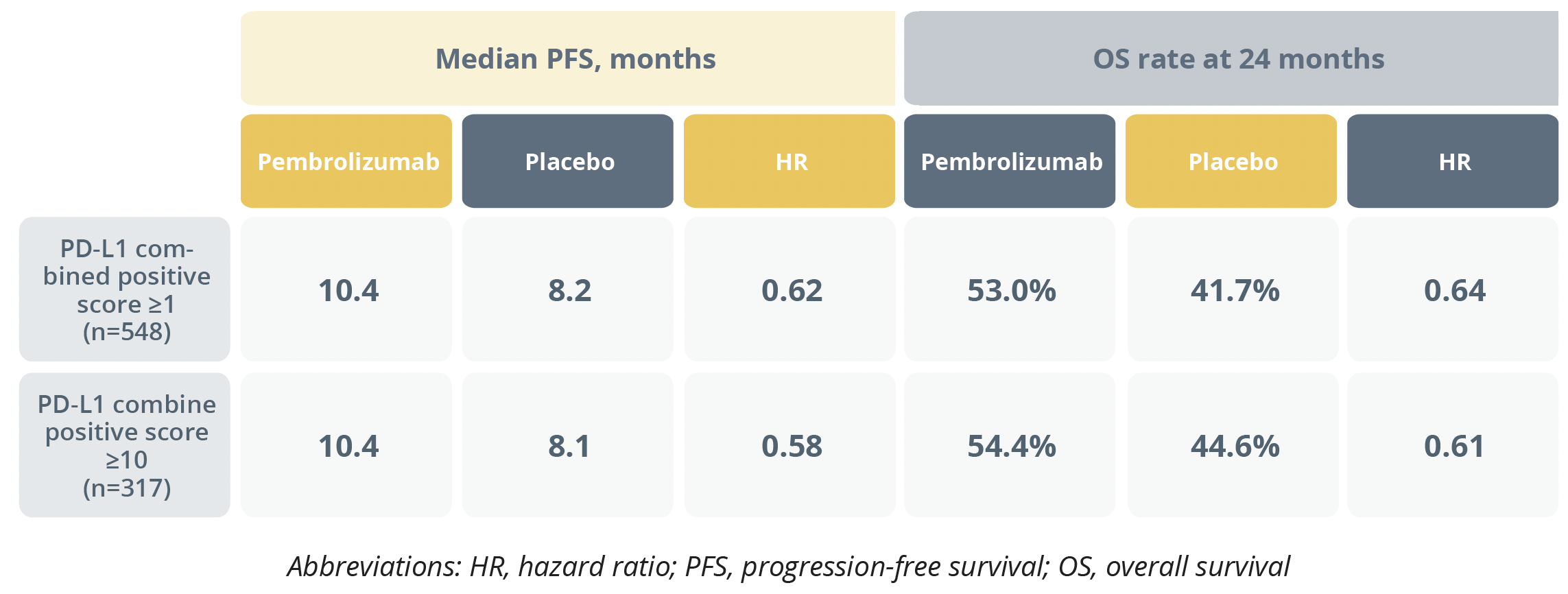
Median progression-free survival (PFS) was 10.4 months with pembrolizumab versus 8.1 months with placebo, and the results were similar when the cohort was stratified by PD-L1 score.
For overall survival (OS), the risk of death at 24 months was a significant 33% lower in the pembrolizumab arm compared with the placebo arm, with estimated OS rates of 50.4% and 40.4%, respectively. Again, the findings were similar regardless of PD-L1 level.
Progression-free and overall survival rates
Columbo said that the safety profile was manageable with pembrolizumab plus chemotherapy and bevacizumab and noted that although individuals who received this regimen had a higher rate of grade 3 or worse treatment-related adverse events than those who received placebo (68.4 vs 64.1%), they also had a longer median treatment duration (10.0 vs 7.7 months).
Discussant Mansoor Raza Mirza, from Rigshospitalet in Copenhagen, Denmark, said the results are “a game changer for these cervical cancer patients where we have not much to offer: this is clearly going to change our standard of care.”
However, based on exploratory subgroup analyses, he said there are more questions to be answered including whether the combination is effective in primary metastatic disease, biomarker negative populations, adenocarcinomas and when given without bevacizumab.
Second-line treatment with cemiplimab superior to chemotherapy for recurrent or metastatic cervical carcinoma
Overall survival duration
In a virtual plenary debate session, Krishnansu Tewari (University of California, Irvine, USA) answered a series of questions about the EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 (NCT03257267) trial that was previously reported at an ESMO Virtual Plenary in May 2021.
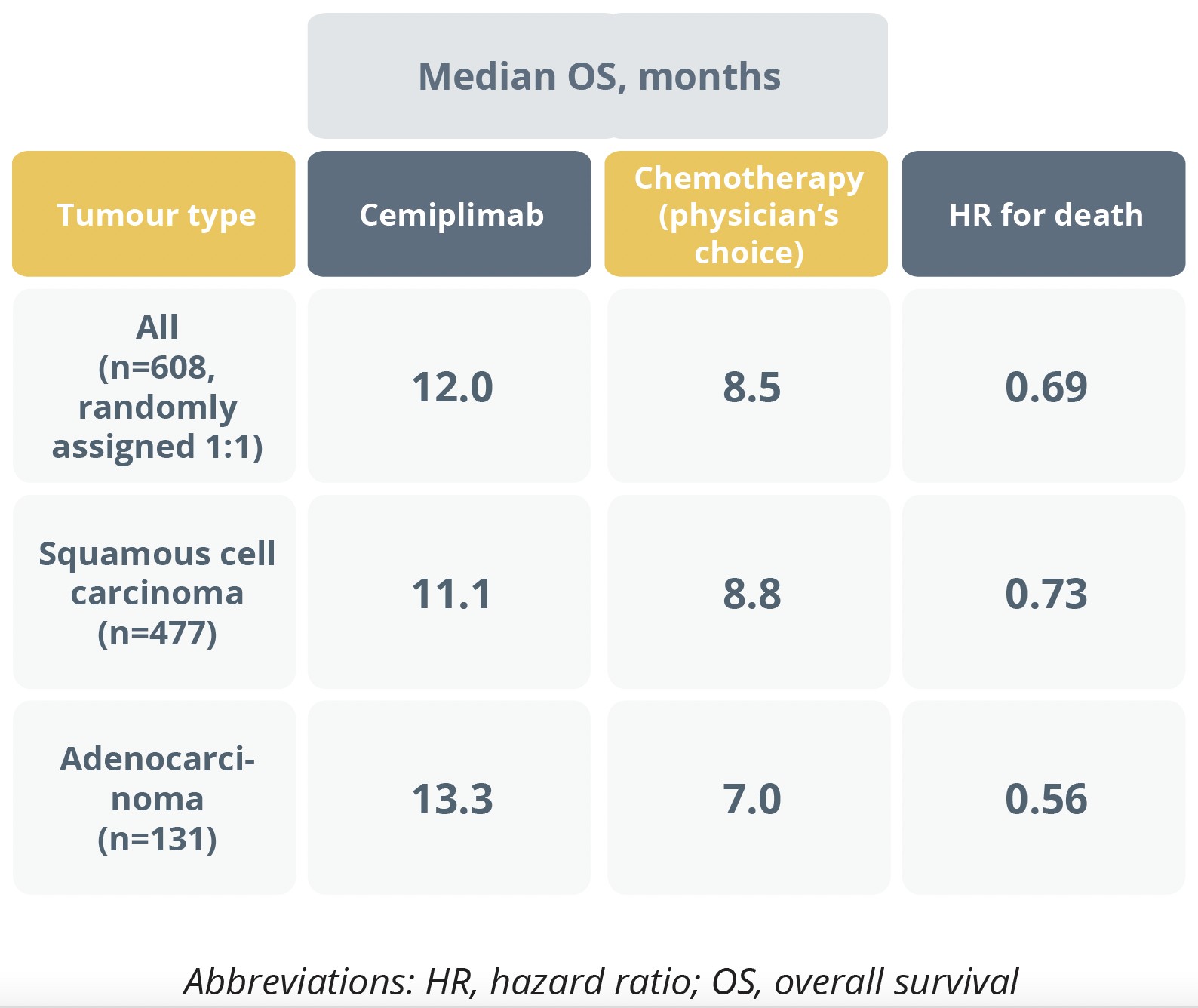
The randomized, phase 3 trial showed that individuals with recurrent or metastatic cervical cancer that had progressed after first-line platinum-based chemotherapy (n=608) had a significant 31% lower risk of death after receiving the PD-1 inhibitor cemiplimab 350 mg every 3 weeks than after physician’s choice of second-line chemotherapy.
However, during the debate session, a participant asked if the EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 findings were now out of date following the publication of the KEYNOTE-826 trial.
Tewari argued that the data are “still very relevant” because although he is excited about bringing checkpoint inhibition into the front-line, there is still “something to be learnt and patients to be helped with our study, especially because it is a monotherapy – it’s not going to have the accumulated toxicity associated with adding platinum, paclitaxel, and antiangiogenesis therapy.”
Ovarian Cancer
Search continues for agent to boost immune checkpoint inhibition in platinum-resistant ovarian cancer
Researchers in the EORTC-1508 trial (NCT02659384) have found no significant PFS advantage when adding atezolizumab with or without acetylsalicylic acid to bevacizumab for the treatment of platinum resistant ovarian cancer, but translational data from the study may help pinpoint the candidates most likely to benefit.
“There remains an unmet need for more effective treatments in platinum-resistant ovarian cancer.”
Susana Banerjee, UK
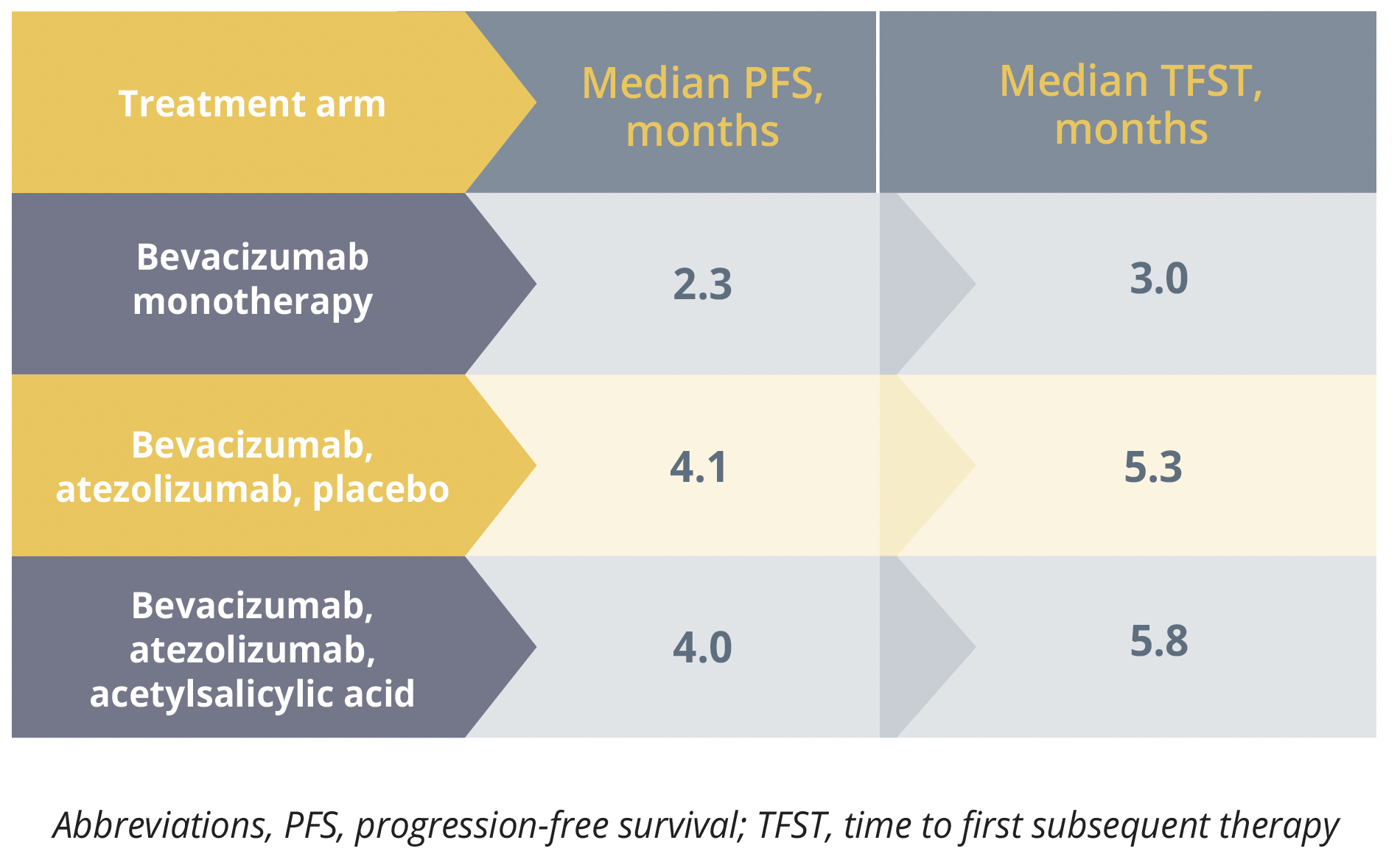
Susana Banerjee, from the Royal Marsden Hospital NHS Foundation Trust in London, UK, reported that, at 6 months, PFS was 24.1% in the first 29 participants randomly assigned to receive bevacizumab 15 mg/kg every 3 weeks. This was not significantly different from the rate of 20.7% among the first 29 participants who received bevacizumab plus atezolizumab 1200 mg and placebo, or that of 27.6% among the first 29 who were given bevacizumab plus atezolizumab and acetylsalicylic acid (320 mg/day).
Progression-free survival and time to first subsequent therapy outcomes
There was, however, a nonsignificant increase in median PFS and a significant increase in time to first subsequent therapy among the participants given combination therapy rather than monotherapy. Banerjee told delegates that time to first subsequent therapy is an “important endpoint in terms of patient management” and this result “may merit further exploration.”
Discussing the findings, Rosalind Glasspool, from the University of Glasgow in the UK, described the study as ambitious, noting that two additional arms (atezolizumab combined with placebo or acetylsalicylic acid) closed early after demonstrating a lack of activity.
She also said the “real strength of the EORTC study lies in the translational work […], which will no doubt produce really important data” that will play a part in unlocking effective immune checkpoint inhibition for ovarian cancer.
Maintenance olaparib rechallenge may delay progression of heavily pretreated ovarian cancer
Results of the phase 3b OReO/ENGOT Ov-38 (NCT03106987) trial revealed that rechallenge maintenance therapy with the PARP inhibitor olaparib significantly reduces the risk of disease progression or death in women with relapsed ovarian cancer who had received at least one previous line of PARP inhibitor maintenance therapy and responded to their most recent line of platinum-based chemotherapy.
ECO in Paris, France, reported that 93% of the 112 women with BRCA-mutated disease and 86% of the 108 with BRCA-nonmutated disease had received three or more previous lines of any chemotherapy.
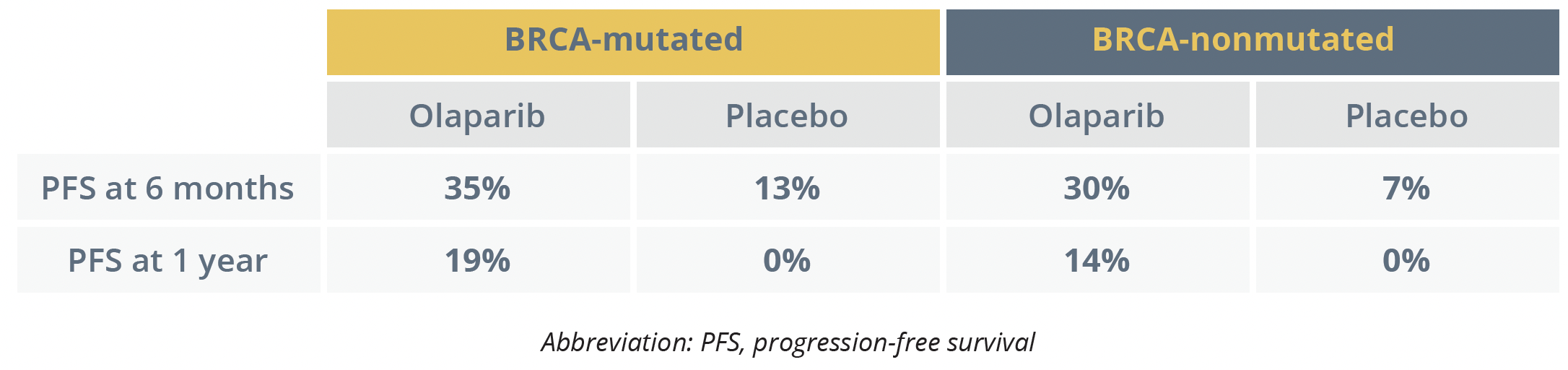
In the BRCA-mutated cohort, median PFS was 4.3 months among the women who were given olaparib 300 mg twice daily compared with 2.8 months among those who were given placebo, corresponding to a significant 43% reduction in the risk of progression or death.
In the BRCA-nonmutated cohort, there was a significant 57% lower risk of progression or death with olaparib versus placebo, with median PFS at 5.3 and 2.8 months, respectively. Pujade-Lauraine noted that, in this group, outcomes were similar regardless of homologous recombination deficiency.
PFS outcomes
Pujade-Lauraine said the adverse event (AE) profile was consistent with that observed during first-line olaparib therapy. Three of 146 participants who received olaparib discontinued therapy due to treatment-emergent (TE)AEs and 26 (17.8%) experienced a TEAE of grade 3 or higher.

©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.

