
Welcome to ESMO 2022
-
The Annual Meeting of the European Society of Medical Oncology (ESMO) was a hybrid meeting, with delegates attending in person for the first time since before the pandemic. ESMO President Solange Peters noted that this made the meeting “feel like a huge success before it has even begun” as the oncology community were able to meet, network and disseminate data in-person in Paris, France. She underlined that, for ESMO, there was never going to be a return to “normal” but was instead progressing forward due to the proactivity that the community has demonstrated in reinventing itself to meet the challenges of our era.

New cancer cases predicted globally by 2030 and 2040
Globally, the ESMO community is already taking action by going the extra mile to comfort patients, to grow as professionals by keeping their knowledge up-to-date, and by raising awareness for cancer prevention. The ESMO community is driven by a shared determination to work towards the best possible outcomes for patients with an inspiring tagline of, ‘Together we can. Together we care.’
Solange Peters then summarised the importance of healthcare sustainability and how our healthcare systems rely on finite resources. She emphasised that a key responsibility of the oncology community should be safeguarding patients’ ability to access high-quality care. ESMO should continue to contribute to the overall sustainability of the healthcare system by nurturing the professional development of oncologists and offering continued support in their day-to-day clinical practice. ESMO’s support and resources should therefore reach every oncology professional.
“As important as doctors’ knowledge and skill is their physical and mental ability to give the best of themselves to patients each day.”
- Solange Peters, ESMO President
Solange Peters then introduced the ESMO 2022 Scientific Co-Chairs, Fabrice André and Charles Swanton. Charles Swanton gave an overview of the impressive proportion of delegates attending ESMO 2022 both in-person and online. He then stated that the purpose of ESMO 2022 is not only a “celebration of getting us all back together”, but also a collaboration between diverse oncology professionals who spend their lives aiming to improving the survival of their patients. ESMO 2022 will focus on “better understanding the disease and treating patients better – from bench to bedside”, which is a matter of time, collaboration, patience and long-term investment. Fabrice André added that current challenges in oncology will be integrated within the ESMO Congress 2022 programme such as early cancer detection and prevention, molecular medicine, and the impact of new drugs assessed in underrepresented patients such as anti-programmed cell death protein-1 (PD1) in the elderly population.

Total participants online and in person
Melanoma
Uveal melanoma
Sapna Patel, Texas, USA, gave an overview of recent treatment developments for metastatic uveal melanoma. In particular, she focused on phase III data of tebentafusp which has demonstrated favourable overall survival (OS) data compared with placebo (21.7 vs. 16.0 months, respectively). She highlighted that a “hallmark feature” and adverse event of interest following treatment with tebantafusp is rash which can be localised or diffuse.
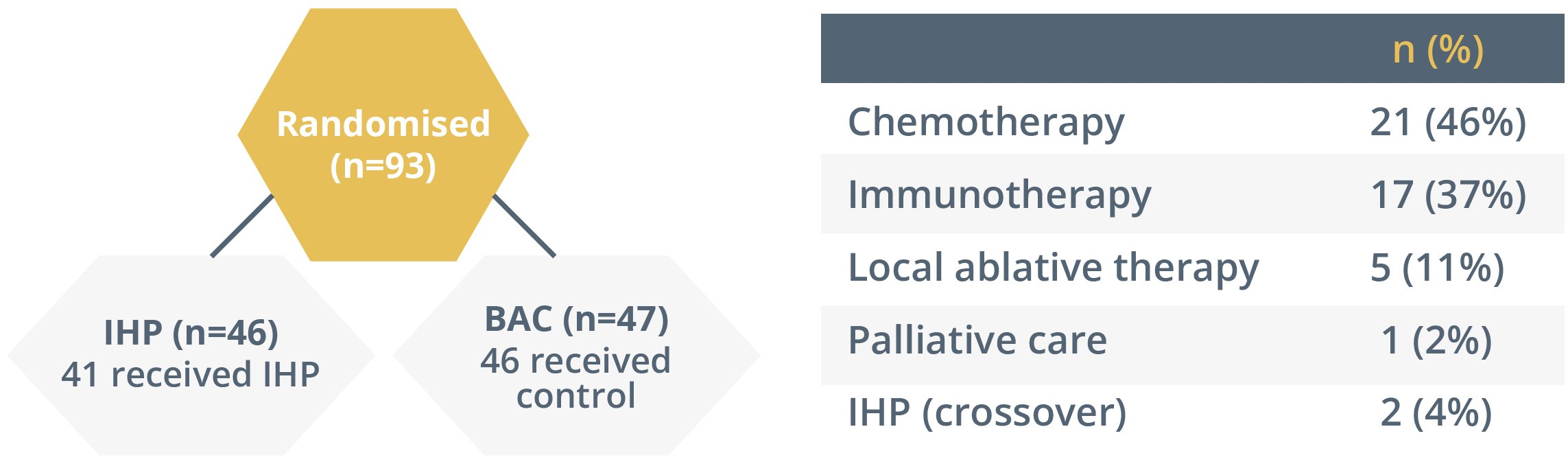
She then summarised the FOCUS trial which assessed the use of immunotherapy in patients with hepatic-dominant ocular melanoma. The study was redesigned to only incorporate percutaneous hepatic perfusion (PHP), as shown below. PHP was associated with a three-times improvement in overall response rate and progression-free survival when compared with best alternative care (BAC).
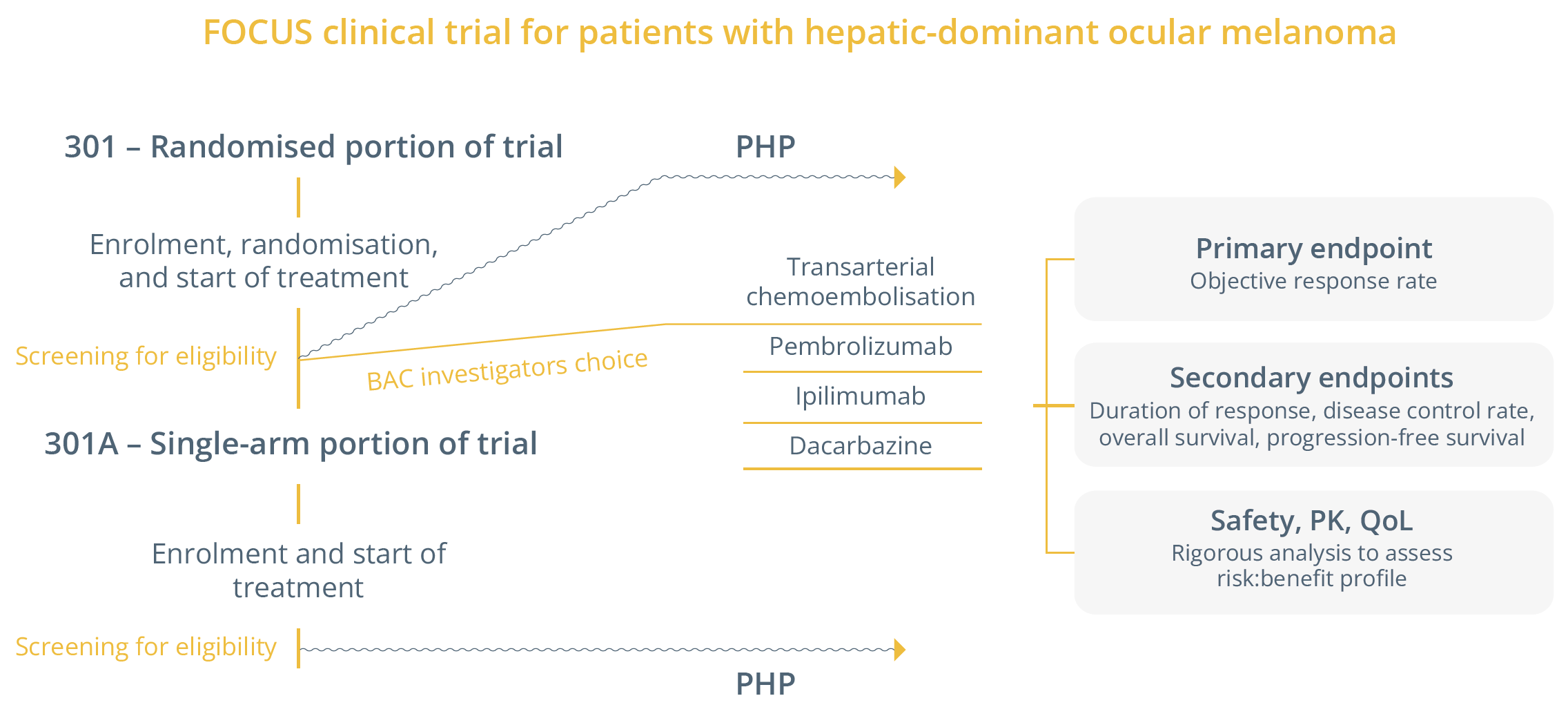
Study design of the FOCUS trial
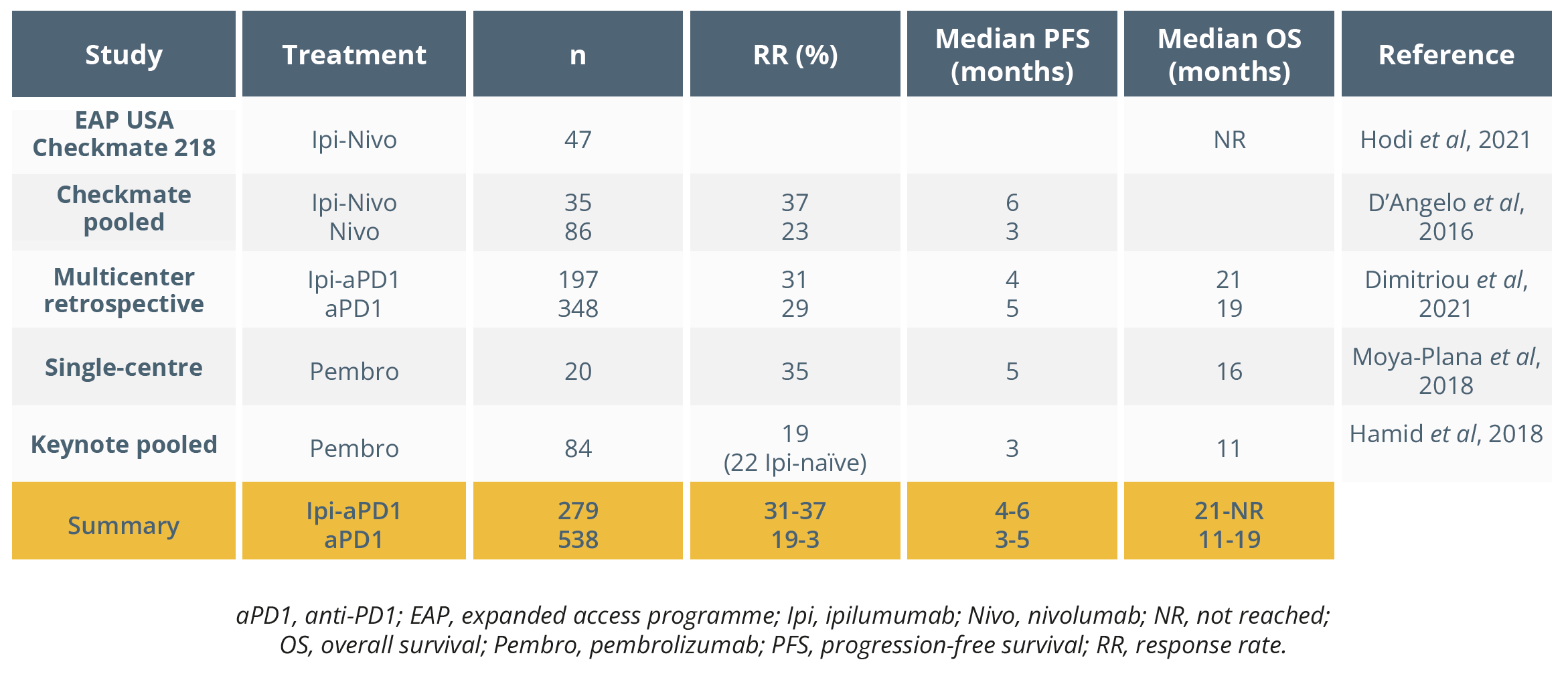
Overview of studies in uveal melanoma
 The SCANDIUM trial was then outlined, which assessed first-line isolated hepatic perfusion with melphalan versus BAC in metastatic uveal melanoma, with an overall response rate of 40% compared with 4.5% (p<0.0001), respectively.
The SCANDIUM trial was then outlined, which assessed first-line isolated hepatic perfusion with melphalan versus BAC in metastatic uveal melanoma, with an overall response rate of 40% compared with 4.5% (p<0.0001), respectively.
“In the last decade, we’ve gone from having very few standard clinical trial options to an abundance of treatments”
- Sapna Patel, Texas, USA
Mucosal melanoma
Primary mucosal melanoma is rare (only 1–4% of melanomas) and occurs in the mucous membrane. Risk factors are unknown as there is currently no evidence for a pathogenic role of chemical carcinogens or viruses. Jessica C. Hassel, Heidelberg, Germany, pointed out that there is no uniformly used classification and staging system for mucosal melanoma. She discussed Poster 801P that described the results of a retrospective analysis of 36 patients (median age of 62 years; median follow-up of 38 months) with resectable mucosal melanoma who received neoadjuvant immune checkpoint inhibitor (ICI) (78% of patients received anti-PD-1 plus anti-cytotoxic T lymphocyte-associated antigen [CTLA]). The primary sites were anorectal (53%), urogenital (25%), head/neck (17%), and oesophageal (6%). Median event-free survival (EFS) was 9.2 months and 3-year EFS was 29%, with a 3-year OS of 55%.
Dr Hassel then summarised the results of Poster 809P that assessed adjuvant anti-PD-1 in patients with resected stage III/IV acral or mucosal melanoma. This was a retrospective analysis of 157 patients (41 [26%] mucosal melanoma; 3 [7%] resected stage IV) and found that 9 (22%) and 19 (46%) either completed therapy or discontinued due to recurrence, respectively (median follow-up of 18 months). She postulated whether there was an obvious benefit from adjuvant therapy in this patient population based on the authors’ conclusions. She then compared the use of ICI therapy in mucosal melanoma, deducing that there was generally no significant advantage of ipilumumab combined with nivolumab (Ipi-Nivo) over anti-PD1 monotherapy (Nivo) based on the studies shown below. She concluded that the use of adjuvant anti-PD1 in this patient population needs further investigation.
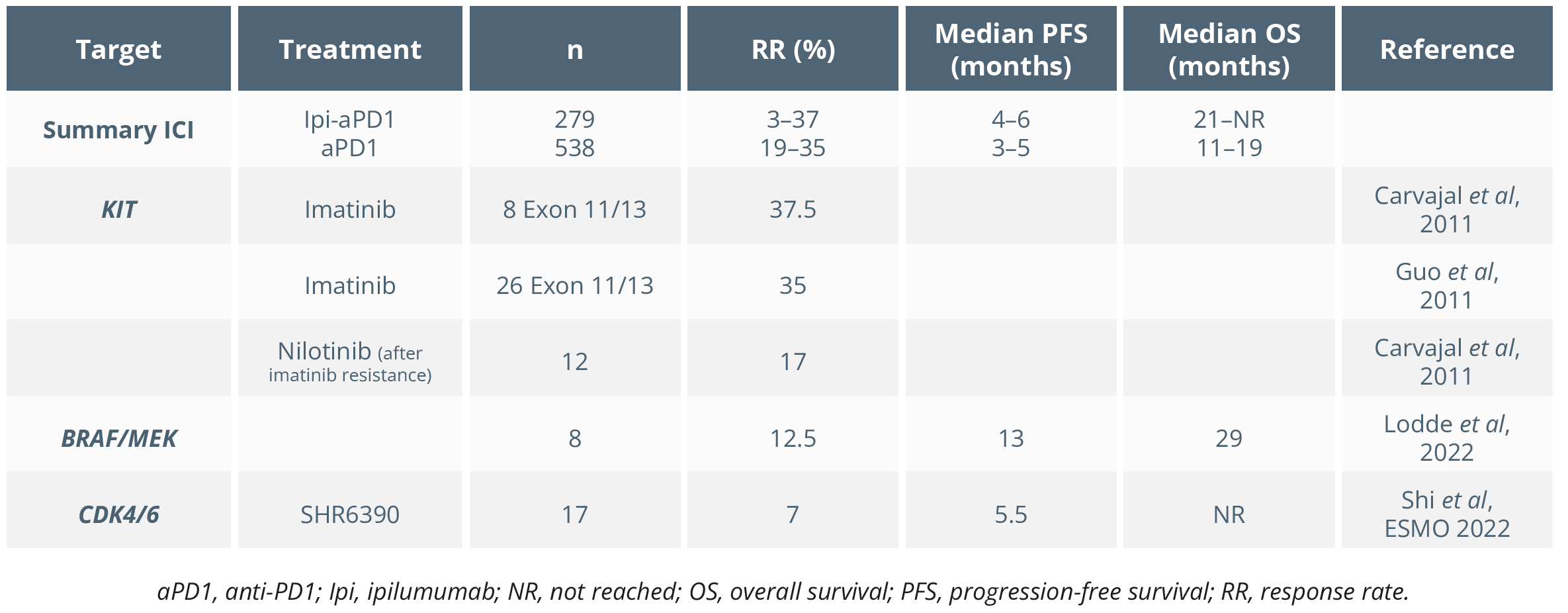
Overview of studies in uveal melanoma
She provided an overview of the mutational landscape in mucosal melanoma, which suggested that the mutational burden was much lower than in cutaneous melanoma. Mutations found in mucosal melanoma are typically KIT, NF1, GNAQ/11, NRAS and BRAF; OS based on mutation status has been shown to be worse in NRAS and GNAQ/11 mutated patients, whereas no difference has been observed in those with KIT and SF3B1 mutations. BRAF mutations have been seen to be frequent in conjunctival melanoma.
Targeted therapies for mucosal melanoma have been shown to achieve durable responses. Dr Hassel compared studies assessing ICI and targeted therapies in mucosal melanoma, as shown below. She concluded that neoadjuvant ICI “might be worth a try in extensive cases”.
She then highlighted that antiangiogenic therapy combined with anti-PDL1 seemed to be associated with high response rates and PFS in mucosal melanoma, as shown below.
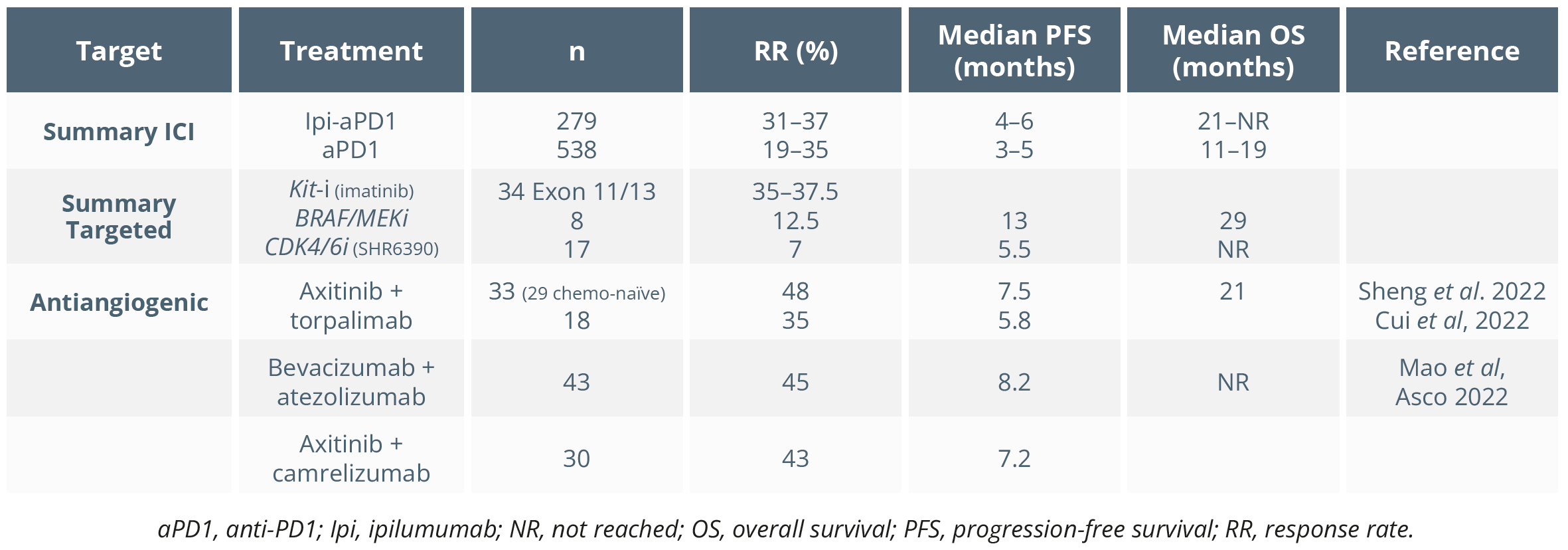
Antiangiogenic therapy combined with anti-PDL1 associated with high response rates and PFS in mucosal melanoma in recent studies
“Maybe antiangiogenic therapies could be useful for the future but, of course, we will need confirmatory trials.”
- Jessica C. Hassel, Heidelberg, Germany
Acral melanoma
Matteo Carlino, Sydney, Australia, clarified that acral melanoma develops from the non-hair-bearing glabrous skin of the soles, palms and nail apparatus. Acral melanoma accounts for a high proportion of melanomas in non-Caucasian populations, with a poor prognosis and significantly low tumour mutational burden. He presented recent studies demonstrating that BRAF/MEK inhibitors had comparable efficacy in acral melanoma and cutaneous melanoma. Checkpoint inhibitor activity has been shown to be lower in acral versus cutaneous melanoma based on the Dutch Melanoma Treatment Registry (NCT04196452; 2058 patients [88 and 1970 acral vs. cutaneous melanoma, respectively]).
Closing Remarks
Following a successful in-person meeting this year with ~23,000 attendees, ESMO 2023 will continue to disseminate the latest cutting-edge data and provide a unique networking opportunity for oncology professionals in-person in Madrid, Spain, from 20–24 October 2023. Save the date for what will be an equally engaging and successful meeting in 2023.