
Welcome to AAIC 2021

Harry Jones, President of the AA, welcomed the delegates to the meeting and spoke of the milestone of finally reaching the very first treatment for Alzheimer’s disease, not just its symptoms, and the potential of having a full new class of treatment and other classes of treatment still to come. He highlighted that this first treatment and others to follow would have had no prospect for approval without the support of the AA over the last 10 years.

TREATMENT
With the recent accelerated FDA approval in June 2021 of aducanumab, this is first new treatment in almost 20 years and the first to target the underlying cause of Alzheimer’s disease (AD). Aducanumab is a second-generation monoclonal antibody and it is indicated for patients with mild cognitive impairment (MCI) or mild dementia.
Development of second-generation monoclonal antibodies to treat AD
In a plenary session, Roger Nitsch, Zurich, Switzerland, provided a brief review of the history of AD discovery and its pathology to the development of monoclonal antibodies to target amyloid-β.
The role of amyloid-β and tau spreading in the acceleration of cognitive decline has been well-defined in AD. Amyloid quantified by positron emission tomography (PET) is visible in prodromal AD and can precede cognitive decline by over 20 years. Initial studies with first-generation antibodies found that they had no effect on amyloid-β plaques. However, the second-generation monoclonal antibodies are selective for aggregated amyloid-β and can reduce the formation of amyloid-β plaques.

Approval status of second-generation monoclonal antibodies for the potential treatment of AD
Aducanumab has been shown to cross the blood-brain barrier, bind amyloid aggregates and attract microglia that remove amyloid-β. Initial clinical studies showed a large degree of amyloid-β reduction resulted in significant improvements across multiple cognitive scales and further studies are ongoing to confirm these results. Studies in smaller patient subsets have also shown effects of aducanumab on tau biomarkers in PET and cerebrospinal fluid (CSF).
Aducanumab is currently the only drug that has been approved for the treatment of AD but others are in phase III development. Aducanumab was approved in June 2021 and donanemab, gantenerumab and lecanemab are in phase III.
Implementation of treatment to practice
The first aducanumb appropriate use recommendations were published at this congress and were discussed in a session co-chaired by Alzheimer’s Association Chief Science Officer, Maria Carrillo, and Jeffrey Cummings, Las Vegas, USA (Cummings et al. J Prev Alzheimers Dis. 2021. In Press).
This session comprised of three panels to talk about three different topics: patient selection; management and monitoring; and patient information.

“The major difference between the PI and our recommendations has to do with proving the presence of amyloid before aducanamab is administered”
Jeffrey Cummings, Las Vegas, USA

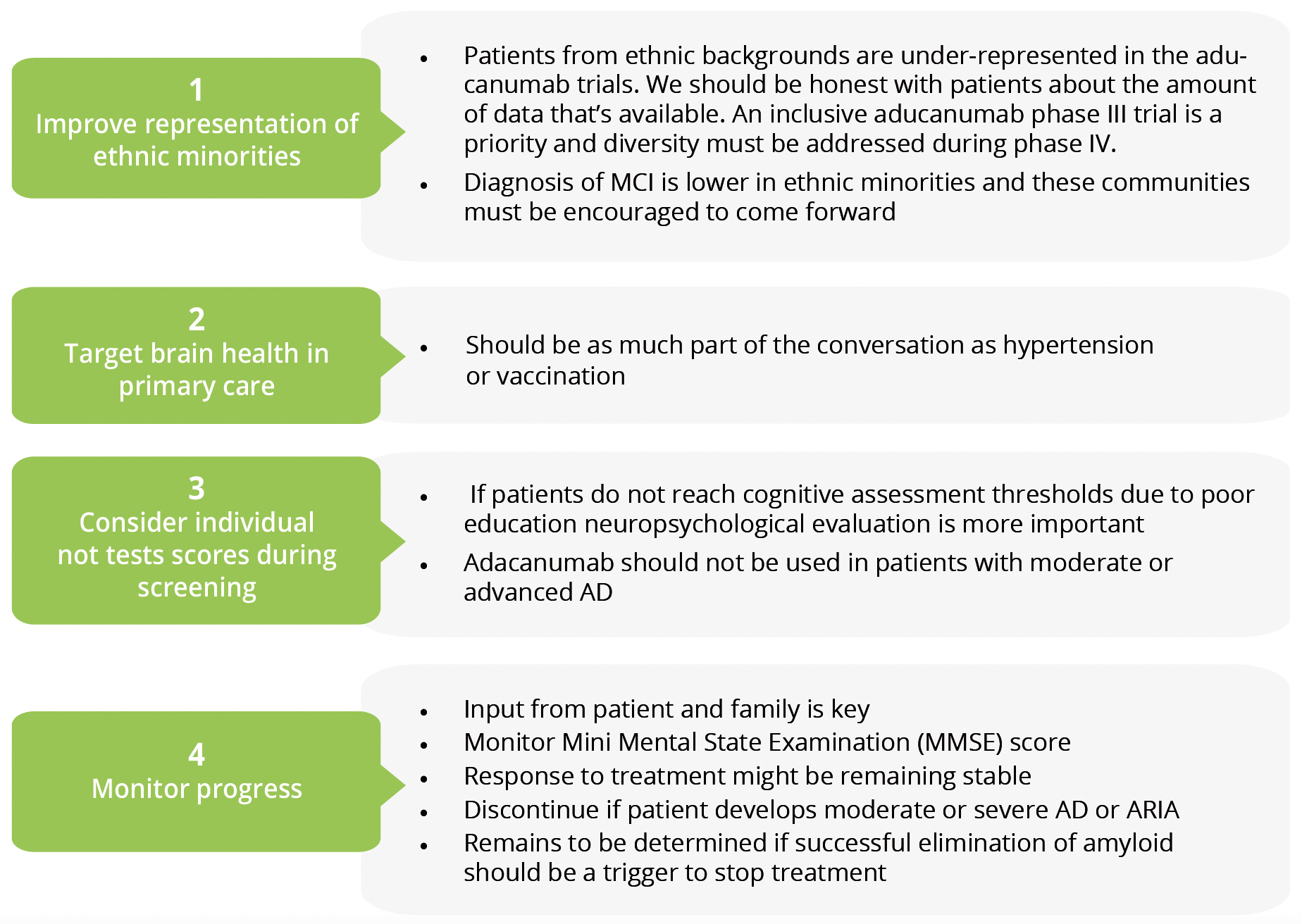
Patient information and discussions
- Diagnosis of MCI is lower in ethnic minorities and these communities must be encouraged to come forward.
- Patients from ethnic backgrounds are under-represented in the aducanumab trials. We should be honest with patients about the amount of data that’s available. An inclusive aducanumab phase III trial is a priority and diversity must be addressed during phase IV.
- Brain health should be as much part of the conversation in primary care as hypertension or vaccinations.
- If patients do not reach cognitive assessment thresholds due to poor education neuropsychological evaluation is more important. Adacanumab should not be used in patients with moderate or advanced AD.
- Monitor Mini Mental State Examination (MMSE) score and use input from patient and family to decide whether to continue or discontinue treatment. Response to treatment might be remaining stable. Treatment should be discontinued if patient develops moderate or severe AD or ARIA. Whether successful elimination of amyloid should be a trigger to stop treatment remains to be determined.
Impact of aducanumab approval on future clinical trials

The availability of an approved disease-modifying agent in AD will raise questions about how trials should be conducted moving forward. For instance, is it ethical to include a placebo group? Should all patients be treated aducanumab as standard, and if so, who should pay for that? How do we design combination trials?
In a session focused on these and similar issues, Kathleen Costello, Chicago, USA, shared her experience from the field of multiple sclerosis in which interferon β-1b was approved as the first disease-modifying agent in 1993. Early challenges were unrealistic expectations, side effects and lack of safety monitoring. However, it is encouraging that research and development in the field has been prolific with now more than 20 approved treatments that use many different modes of action, and no appearance of any waning of interest for research.

David Knopman, Rochester, USA, considered that observational research is still relevant given the availability of disease-modifying agents and suggests that there are two important implications. Firstly, a clinical diagnosis of MCI or mild dementia at the outset of a trial or emerging during a trial should be fully disclosed to patients. Secondly, brain amyloid-β status must be determined, preferably by quantitative assessment. Amyloid-β status should generally be disclosed to inform participants that they are in a group designated as potential candidates for aducanumab, but this requires adequate counselling capability to discuss what this means in terms of risk of future decline.
The speakers agreed that the biggest issue in future trial design is the validity of including a placebo/control group. Joshua Grill, Irvine, USA, considered that they currently remain ethical since it is not yet established as a new standard-of-care but the field will need to pay close attention to dropout rates and recruitment to ongoing and new trials. Placebo-controlled is the option that provides the optimal efficiency and validity, but it risks delaying benefits of approved therapy (Option 1). Alternative options would provide valuable information for clinical practice, but carry risks of drug interactions (Option 2) and potentially challenges to validity (Options 2 and 3). Option 1 would compare the new drug vs placebo; Option 2 would allow the approved drug as background therapy throughout; and Option 3 would recognise the approved drug as standard-of-care and be of equivalency or superiority design.

Options for future clinical trial designs
“It is unlikely that a new treatment will immediately become standard-of-care and make placebo-controlled trials unethical”
Joshua Grill, Irvine, USA

Adjunctive or factorial clinical trial designs
Reisa Sperling, Boston, USA, posed the question of the need for individuals to become amyloid negative before starting tau therapy. It should also be considered whether to continue anti-amyloid treatment and/or amyloid biomarker monitoring throughout the trial. Studies could combine anti-amyloid and anti-tau using either adjunctive to amyloid treatment or factorial design. Adjunctive studies would enrol participants on stable anti-amyloid dosing whereas a factorial design would rely on a potentially complex dosing sequence.
IMPACT OF COVID-19 ON AD PRACTICE AND POLICIES
The COVID-19 pandemic has been devastating for older residents with dementia, not only the risk of serious illness and mortality from the virus but also the detrimental effect of the social distancing measures on their physical and emotional well-being. Many patients with dementia depend upon on daily socialisation and engagement to maintain their cognitive health and cannot understand this unfamiliar world of tests, isolation and social distancing.
Perspectives from long-term and community-based settings

A session chaired by Douglas Pace from the Alzheimer’s Association invited representatives from the residential home care industry to speak about the immediate and longer-term approaches taken by their companies in response to the pandemic. Early in the pandemic, the Dementia Care Provider Roundtable, led by Dr Pace, issued COVID-19 related guidance on caring for persons with dementia, which was supported by 36 organisations and affiliated associations.

Services that can be provided to AD patients in their own homes

In addition to reactive changes to improve infection control, Patrick Doyle from the John Hopkins School of Nursing described long-term changes that can be adopted, for example, holistic hand massages that also act to deep clean hands and sanitising with annual applications of long-lasting disinfections and changes to filtration systems. Other long-term changes have improved connectivity to the outside community with in-apartment technology and expansion of telemedicine services. The key to overcoming social isolation is to promote participation and togetherness in a virtual world.

Immediate and long-term changes to improve infection control
Use of telehealth for cognitive assessment

Pandemic-related restrictions have created a need for at-home cognitive assessment tools and this was the focus of another session. Research presented by Munro Cullum, Texas, USA, showed that in-clinic teleneuropsychology, i.e. neuropsychological tests to evaluate cognitive status via videoconference technology, is as reliable for measuring a battery of cognitive tests as in-person evaluation. However, he warned that the risk of distraction in the home environment could threaten its validity.

“In the USA, Alzheimer’s and dementia-related deaths have increased by 16% during the COVID-19 pandemic”
Katherine Dorociak, Minneapolis, USA

Katherine Dorociak, Minneapolis, USA, reported results from the Survey for Memory, Attention, and Reaction Time (SMART), which is a 2-year longitudinal study in older adults who are cognitively intact or with MCI. Participants were asked to complete the short, self-administered web-based assessment every month. Executive functioning tasks, including the Trails B completion time and the Stroop Click Count, were the most sensitive indicators of cognition. Total completion time and time of day of test completion may also provide value.

In a cohort of older aboriginal Australians, Kylie Radford, Sydney, Australia, tested in-person vs telephone versions of the MMSE and the Kimberly Indigenous Cognitive Assessment-screen (KICA-screen), which is recommended for use with Aboriginal and Torres Strait Islander patients. Preliminary data suggest that the telephone version may be a suitable alternative for the MMSE but that the KICA-screen requires further validation.

Rebecca Street, London, UK, shared her experience of remote testing the Insight 46 population, a British 1946 Birth Cohort of the longitudinal neuroscience MRC National Survey of Health and Development. They demonstrated the feasibility of teleneuropsychology but found that concerns about technology was the major reason for drop-out despite offering one-to-one videoconferencing training.
Policy changes
Another session discussed how policies relating to those with AD and related dementias (ADRD) have changed in response to the pandemic in different countries around the world.
Grace Yoon, Taipei, Taiwan, explained that the main spike of COVID-19 infections in Taiwan occurred in early 2021 and resulted in clusters of cases in long-term facilities. National public health policies quickly came into place to curb the spread of COVID-19 infection in elderly care settings, impacting dementia care. Actions of patient advocacy movements built on existing knowledge to change practice and policy. Patient advocacy groups have played an important role in providing resources and support to the dementia community, for example, releasing online videos to teach patients how to protect themselves and others during the pandemic.

Action of advocacy groups has led to changes in practice and policy

Australia has maintained a low incidence of COVID-19 with border closures, quarantine rules and strict lockdown policies. However, Briony Dow, Melbourne, Australia, explained how ill-prepared aged care facilities were in terms of staff training, resources and integration between health and aged care systems. A big issue was the spread of infections by underpaid staff needing to work a range of jobs to make a living wage. This led to policies related to improved funding within this sector as well as improved training and well-being support. A major reform of aged care system in Australia has been announced.
“The COVID-19 pandemic caught the aged care sector unaware and brought to light a range of longstanding problems”
Briony Dow, Melbourne, Australia

There has been a heavy toll of the pandemic in the USA with an estimated 25–30% of infections and deaths among people living with ADRD. Walter Dawson, Oregon, USA, explained that the primary blame has been assigned to the Long-Term Services and Supports (LTSS) system but poor public health policy and long-term disinvestment at the federal level have now become apparent. Immediate reforms will help but comprehensive reform is essential.
BIOMARKERS
The topic of biomarkers for Alzheimer’s disease were the focus for a number of sessions at this year’s congress. Biomarkers of disease pathophysiology are important for staging and subtyping and are frequently detectable over a decade before the onset of clinically-relevant cognitive symptoms. There has been an expansion of the portfolio of AD biomarkers in recent years that has added to the established biomarkers of amyloid-β and tau. Emerging biomarkers are bodily-fluid based and measure glial and synaptic activation, and neurodegeneration.
Biofluid-based biomarkers for AD

Biofluid-based biomarkers was the topic of a plenary talk by Henrik Zetterberg, Gothenburg, Sweden. Originally, CSF and plasma tau concentrations were thought to reflect neurodegeneration and tangle pathology. However, translational biomarker work has made it clear that tau is released from neurons in an activity-dependent manner, which is stimulated by amyloid-β plaque pathology. The latest innovations in amyloid pathology are the use of plasma amyloid-β assays, assays for brain-derived amyloid-β and the development of fully automated clinical chemistry tests.
Neurofilament light (NfL) can be measured in CSF and plasma or serum. It is a dynamic biomarker of neurodegeneration and neuroaxonal injury and can be detected across many neurodegenerative diseases. It has been shown to be predictive of disease progression in MCI. Levels can increase with active neurodegeneration or ongoing injury and decrease with drugs that slow or stabilise neurodegeneration. Other promising areas are amyloid-β, tau and glial biomarkers of inflammation in CSF and blood, and synuclein pathology markers in CSF.
The blood biomarkers will be useful as early diagnostic tests to determine which patients are likely to benefit from disease-modifying treatment but there’s a need for more data in diverse populations.
The ever-changing landscape of AD biomarkers
In a scientific session, Melissa Murray, Minnesota, USA, explored potential neuropathic strategies to validate AD biomarkers. Regional AD phosphorylated tau (P-tau) can be visualised and quantified using PET. These biomarkers have been validated with neuropathology of AD by PET-to-autopsy studies and autoradiographic studies in the post-mortem brain tissue. Recent studies have also shown good correlation between levels of p-tau231 and p-tau181 in plasma and global measures of disease.

Current biomarkers in AD research
Thomas Karikari, Gothenburg, Sweden, talked further about using biofluid-based biomarkers. CSF and plasma P-tau reflect amyloid-β-induced neuronal tau phosphorylation and secretion, which in turn may be predictive of future neurodegeneration and tangle pathology. Aβ42/Aβ40 ratio in the cerebrospinal fluid (CSF) reflects amyloid-β accumulation in the brain and is the fluid biomarker with the firmest correlation with brain pathology.
“Initial amyloid-β and tau changes are detectable over a decade before the onset of clinically-relevant cognitive symptoms”
Susan Landau, Berkeley, USA
Susan Landau, Berkeley, USA, reviewed the use of PET imaging biomarkers in AD. PET reflects insoluble, fibrillar forms of amyloid-β/tau accumulation. This technology can be used to detect early, regionally-specific changes that predict downstream neurodegeneration and cognitive decline, offering an opportunity for therapeutic intervention. The early signal is clinically relevant and standardised acquisition/analysis is feasible. However, there are a number of technical and cost considerations for successful implementation of PET relative to other biomarkers in research, trials, and clinical practice, particularly where fluid biomarkers could be sufficient. Challenges include a limited ability to measure non-amyloid-β/tau pathology, weakness of PET-fluid relationships in the earliest phases of AD, and off-target binding.

Unique features and challenges of PET imaging biomarkers in AD

Biomarkers could be used to improve the efficiency in secondary prevention clinical trial design. Michael Schöll, Gothenburg, Sweden, described how tau biomarkers could be used to increase the likelihood of participants meeting endpoints and reducing patient burden. Screening and enrichment could recruit participants that are on the AD trajectory and are likely to exhibit regionally relevant longitudinal increases in tau. As illustrated, blood p-tau screening decreases screening failures and increase relevant positive biomarker prevalence; PET-/CSF-based enrichment maximises target specificity; a PET-based baseline establishes regional and quantifiable baseline; and a PET-based outcome assesses regional and quantifiable change.

Use of biomarkers to improve efficiency of clinical trial design
Should we disclose biomarker and neuroimaging results in symptomatic patients?

With the availability of AD biomarker information, it is important to understand whether result disclosure is positive for patients and their families in that it reduces doubt about the diagnosis or if it has the unintended consequence of closing the door on hope. Jennifer Lingler, Pittsburgh, USA, discussed this important topic in a plenary session. Post-disclosure distress has been observed in symptomatic patients from trials undergoing amyloid PET but it has not translated to clinically actionable depression or anxiety. Relief was the most commonly reported emotional state, and this was the case in both amyloid negative and positive cases. Patients and their families often feel that the value of knowing the amyloid PET scan results validates the cognitive changes they suspected and provides a basis for making medical and lifestyle decisions to plan for the years ahead. The evidence suggests that there is no reason to view biomarker testing as more impactful than genetic testing in asymptomatic people or dementia evaluations in patients. However, there is a need for more research in more diverse populations to understand who may be at particular risk of distress and/or ‘scananxiety’ (the distress before, during or after a scan to diagnose disease or monitor recurrence/progression). Best practice guidelines will be required in this field as testing expands to tau PET and plasma-based biomarkers.
Use of biomarkers to improve efficiency of clinical trial design
This session focused on non-neuroimaging biomarkers that are being studied in the context of AD. Non-neuroimaging biomarkers include spatial disorientation, speech, functionality, and brainwave activity.

Potential non-neuroimaging biomarkers in AD
Spatial disorientation: Doreen Goerss, Rostock, Germany, explained that wearable sensors can be used to measure activity and vital signs continuously and unobtrusively in elderly people. They have investigated whether sensor data could be used to detect functional changes or specific behaviours that are linked to AD. Individuals with dementia were asked to follow a 150 m route around the clinic while the path that they took was mapped using beacons and a smartwatch. This technology demonstrated a high rate of spatial disorientation and may be a useful, non-invasive tool to help with diagnosis.
Speech: Nicklas Linz, Saarbrücken, Germany, explained how speech analysis might be useful tool to detect cognitive and emotional changes in AD. Different speech tasks are used to measure different functions. In this study, samples of speech from patients at different stages of AD, including subsets with apathy and depression were analysed automatically and by hand. Both techniques were highly accurate in demonstrating speech differences based on clinical diagnosis. Speech and language features represent a promising biomarker candidate as they can be automatically and remotely extracted, even over the telephone.
Functionality: Marijn Muurling, Amsterdam, The Netherlands, reported on the Remote Assessment of Disease and Relapse – Alzheimer’s Disease (RADAR-AD) study which uses remote monitoring techniques to monitor functionality. Healthy controls and individuals with preclinical AD and prodromal AD were asked to complete augmented reality tasks through an app that recreates an activity of daily living requiring spatial navigation and memory. It provided an outcome of Neuro Motor Index (NMI). This score was lower in both preclinical and prodromal AD groups compared with healthy controls. Since there was no difference between the groups in traditional cognitive and functional tests NMI may be more sensitive than standard clinical tests in detecting the early stages of AD. The full dataset is expected June 2022.
Brainwave activity: It is known that left-frontal MCI-like brainwave patterns are associated with slowed memory retrievals. Yang Jiang, Kentucky, USA, investigated whether so-called ‘senior moments’ – a brief delay in memory – could be related to a change in brainwave activity. They used traditional scalp electroencephalogram (EEG) headsets to record brain activity at rest and during a working memory task in healthy older adults. ‘Senior moments’ were time intervals of 1–2 seconds, during which participants’ memory performance dipped, with slower reaction times and less accuracy. EEG traces from tasks with a slightly delayed but accurate memory response showed MCI-like signatures in their left-frontal sites compared to traces from tasks for which response was both accurate and fast. This might offer a quick, non-invasive and affordable pre-screening tool for predicting cognitive decline in a clinical setting.
Closing remarks
The next AAIC will be held in San Diego, USA from July 31–August 4, 2022.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.