Welcome to 2021 ASCO Annual Meeting

Lori J. Pierce, 2020–2021 ASCO President
The 2021 ASCO Annual Meeting, now in its 57th year, took place under a virtual format for a second year on 4–8 June 2021 due to the ongoing impact of the COVID-19 pandemic. This year’s presidential theme was ‘Equity: Every Patient. Every Day. Everywhere,’ which aimed to highlight the importance for oncologists to use this moment of cancer progress to be certain that health equity in cancer care becomes a reality for all patients. Opening the meeting, ASCO President Lori J. Pierce highlighted this year’s focus on a critical safe path forward and asked attendees to identify ways to ensure that all patients can access and benefit from the latest cancer advances and high-quality cancer care. Dr Pierce noted that over the past year, the COVID-19 pandemic has highlighted the pervasive and unjust health inequities that exist all over the globe. Whether barriers result from geography, race/ethnicity, age, sexual orientation and gender identity, health insurance, culture, or trust – or all of these, she added that physicians have a responsibility to meet them head on.
“Together, we need to confront and address complex forces and systems that have created disparities in cancer care, treatment, and research.”
Lori J. Pierce, 2020–2021 ASCO President

Lung cancer
Treatment
A growing number of targeted therapies are available for use in patients with metastatic non-small cell lung cancer (NSCLC). However, it remains important to consider the presence of specific mutations and genetic alterations in order to determine whether a targeted therapy is appropriate on an individual patient-by-patient basis. Thus, molecular testing is now the standard of care to detect the presence of genetic variants and determine which patients are eligible for a targeted therapy. A number of challenges remain in selecting the most appropriate therapies and understanding the various types of molecular tests and pharmacologic characteristics of targeted therapies. Christine M. Lovely, Vanderbilt University Medical Center, Nashville, USA, explored specific issues in using molecular testing to guide treatment decisions by summarising treatments given to three patients with metastatic NSCLC for whom next generation sequencing (NGS) of a large panel of genes and programmed death-ligand 1 (PD-L1) immunohistochemistry were undertaken.
“Biomarker testing in lung cancer will become increasingly complex in the future.”
Christine M. Lovely, Vanderbilt University Medical Center, Nashville, USA
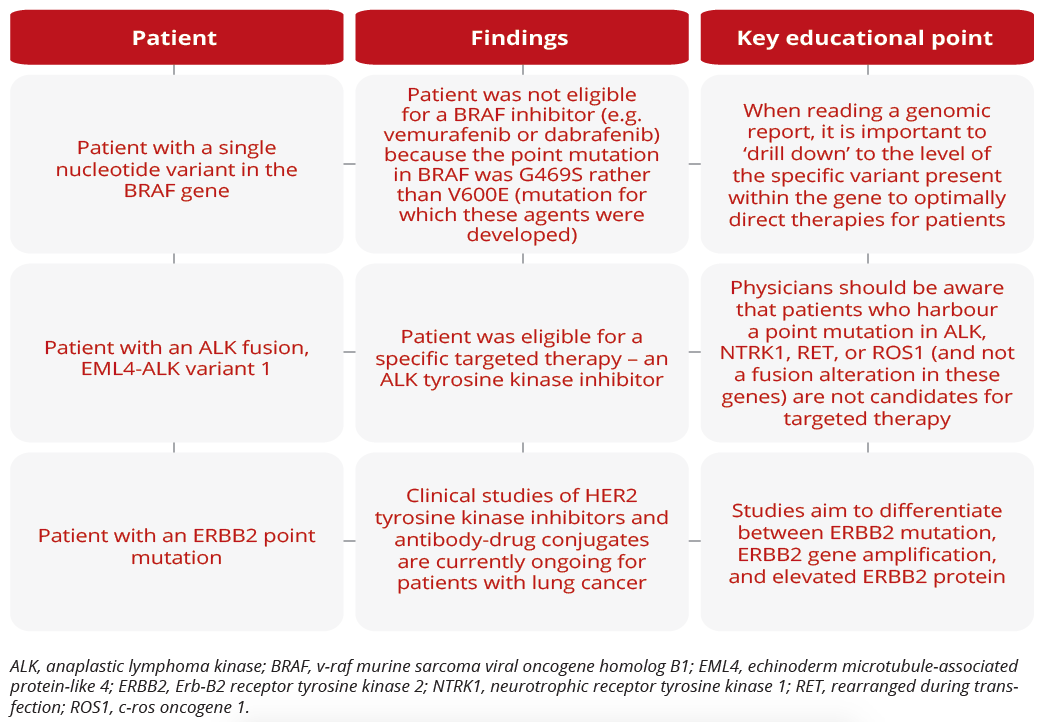
Treatment decisions for three separate patients
Dara L. Aisner, University of Colorado School of Medicine, Denver, USA, discussed types of molecular testing, noting that NGS is the first platform to offer both clinical sensitivity and improvements in analytic sensitivity compared with Sanger sequencing. Within the NGS platform, there are many different types of tests that vary in which genes they probe and the types of genetic alterations they can detect. While a targeted mutation assay assesses mutations in a defined set of genes (from several to several hundred genes), exome testing includes all coding genes. However, neither test type typically detects gene fusions, which play an important role in lung cancer. In contrast, transcriptome testing probes all genes expressed in a sample and typically detects gene fusions.
“Look for the word ‘fusion’ instead of terms such as ‘rearrangement’ and ‘translocation’ in test reports, because fusions lead to functionally active transcripts that may be targetable with therapy.”
Dara L. Aisner, University of Colorado School of Medicine, Denver, USA
Targeted therapies for NSCLC span a diverse array of pharmacologic characteristics. R. Donald Harvey, Winship Cancer Institute of Emory University, Atlanta, USA, explored the MET inhibitors capmatinib and tepotinib, and the RET inhibitors selpercatinib and pralsetinib, all of which have recently been approved for use in NSCLC. Of note, resistance to these therapies can develop via mutations within the kinase domain or through activation of escape pathways. A novel class of drugs - proteolysis-targeting chimeras (PROTACs) - mark targets for degradation by combining a molecule that binds with the target and an E3 ubiquitin ligase protein. While no clinical data are currently available, data from laboratory-based studies suggest that afatinib- or gefitinib-based PROTACs may be active in EGFR-sensitive cells.
“PROTACs hold promise as we consider the next generation of therapeutics in NSCLC.”
R. Donald Harvey, Winship Cancer Institute of Emory University, Atlanta, USA
Maximilian Diehn, Stanford University School of Medicine, Stanford, USA, discussed the use of liquid biopsies for the personalised management of early-stage NSCLC and highlighted that detectable circulating tumour DNA (ctDNA) pre-treatment may be a useful biomarker for selecting patients for neoadjuvant therapy. In addition, ctDNA analysis can robustly identify post-treatment minimal residual disease (MRD) in patients with localised lung cancer, identifying residual/recurrent disease earlier than standard-of-care radiologic imaging, allowing the facilitation of personalised adjuvant treatment at early time points when disease burden is lowest.
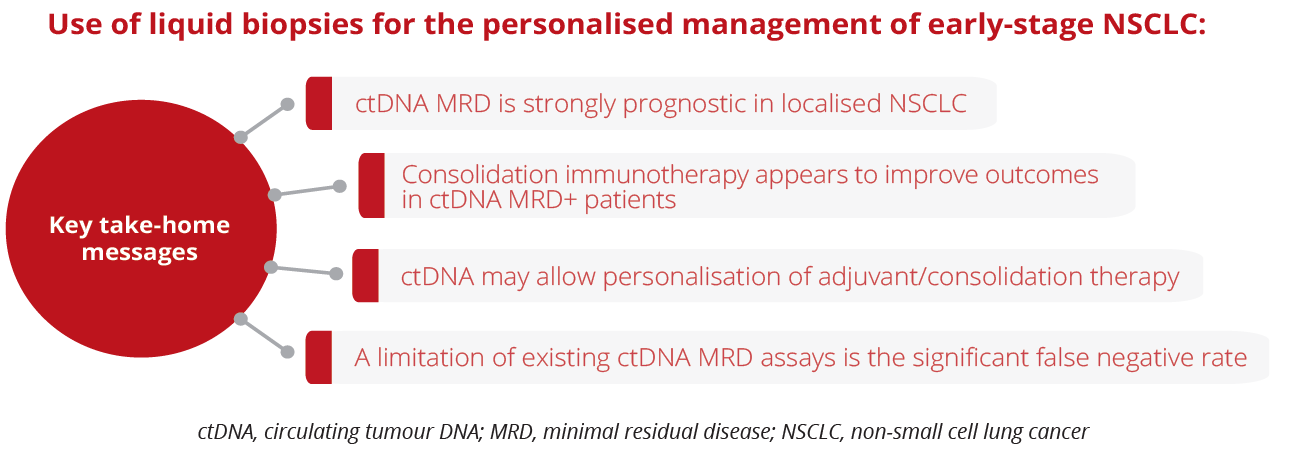
Use of liquid biopsies for the personalised management of early-stage NSCLC
“Lack of a ctDNA response early on during consolidation immunotherapy is associated with poor clinical outcomes.”
Maximilian Diehn, Stanford University School of Medicine, Stanford, USA
Ibiayi Dagogo-Jack, Massachusetts General Hospital, Boston, USA, explored new neoadjuvant and adjuvant approaches for localised NSCLC. Most patients with localised lung cancer who are treated with current curative intent will ultimately develop metastatic disease, highlighting the necessity of developing alternative perioperative approaches. Dr Dagogo-Jack presented data to show that preoperative administration of a PD(L)1 inhibitor is feasible and associated with a major pathology response rate of approximately 20–45%. Of note, there are several ongoing phase III studies evaluating the use of checkpoint inhibitors in the adjuvant setting. In addition, there are multiple studies exploring perioperative targeted therapy and results from these studies are eagerly awaited.
“The addition of chemotherapy to checkpoint inhibitors increases the degree of pathologic regression.”
Ibiayi Dagogo-Jack, Massachusetts General Hospital, Boston, USA
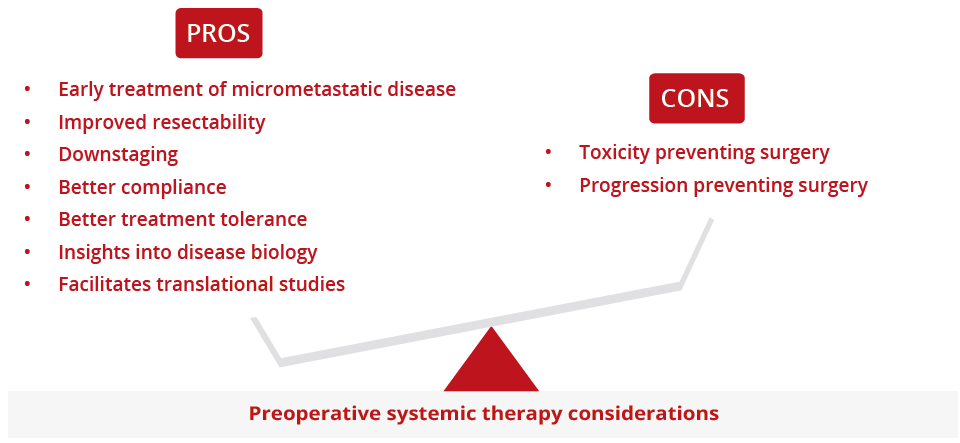
Pros and cons of preoperative systemic therapy
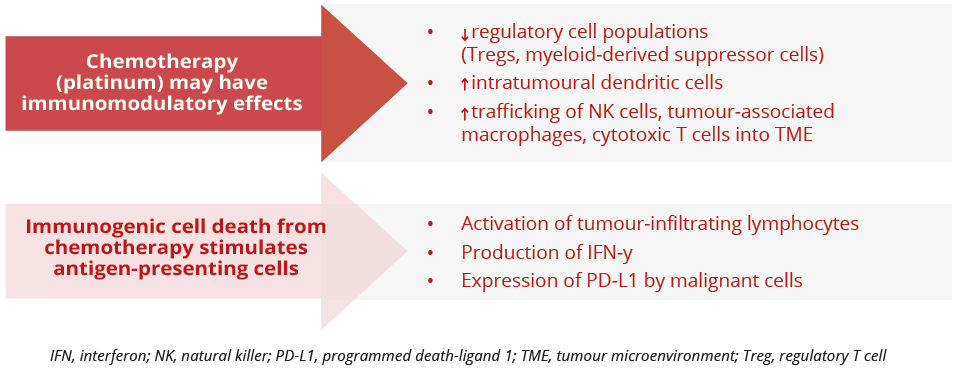
Potential immunomodulatory effects of chemotherapy
Closing Remarks
As a virtual event for the second year running, the 2021 ASCO Annual Meeting continued to provide the unique and unparalleled opportunity to connect one of the largest, most diverse audiences in global cancer care, along with enabling wider access to ground-breaking science and practice-changing research in oncology, the latest trends in clinical application, and treatment and insights on equitable cancer care. It is expected that more than 42,000 delegates from 138 countries will have accessed content during this year’s meeting.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.