
The Annual Meeting of the European Hematology Association (EHA), now in its 25th year, took place under a new virtual format on 11–21 June 2020. Opening the meeting, EHA President John Gribben highlighted that this year’s theme was ‘Unfolding the future!” and although the COVID-19 pandemic changed the format of this year’s EHA 2020 – and renamed it to ‘EHA25 Virtual’ – the congress remained the leading meeting place for haematologists in all fields of this specialty within Europe and beyond.

John Gribben, EHA President
Professor Gribben highlighted that this year’s interactive virtual format still enabled delegates to immerse themselves in the latest news in haematology and its subspecialties. Delegates had access to clinical and translational research data of new approaches to diagnosis and treatment, including information on innovative techniques, diagnostic tools and risk-assessment strategies.
“This year’s virtual meeting will deliver haematological innovations and evidence-based knowledge of primary clinical relevance.”
John Gribben, EHA President
Leukaemia
Genetics
Infectious stimuli are believed to play a major role in the aetiology of the most common types of acute lymphoblastic leukaemia (ALL), but the critical determinants leading to oncogenesis in children are unknown. Ongoing research aims to understand the mechanism by which natural exposure to common infections triggers the disease, with the ultimate goal of identifying potential preventive strategies.
Carolina Vicente-Dueñas, Institute of Biomedical Research of Salamanca, Salamanca, Spain, presented data which demonstrated that an intact gut microbiome protects genetically predisposed mice against leukaemia (Abstract S100). In contrast, microbiome disturbance by antibiotic treatment early in life was sufficient to induce leukaemia in predisposed mice even in the absence of an infective environment. In the presence of infectious stimuli, antibiotic treatment increased the incidence of precursor B-cell ALL incidence in predisposed mice from 22% to 63%. Thus, these data suggest that the risk of developing leukaemia may be reduced by modulating the gut microbiome early in life.
“Gut microbiome deprivation via antibiotic treatment early in life is a risk factor for leukaemia development.”
Carolina Vicente-Dueñas, Institute of Biomedical Research of Salamanca, Salamanca, Spain
Genome-wide technologies used to discover novel genetic aberrations in acute myeloid leukaemia (AML) to guide treatment
Genome-wide technologies to discover novel genetic aberrations in patients with AML
Treatment
Haematopoietic stem cell transplantation (HSCT) may offer the chance of a cure for patients with leukaemia and other blood cancers, but the process of conditioning the body to receive such a transplant can be harsh, involving total body irradiation (TBI) as well as chemotherapy conditioning. However, Christina Peters, St. Anna Children’s Hospital, Vienna, Austria, presented data from the global FORUM (For Omitting Radiation Under Majority Age) study which demonstrated that both steps are needed in paediatric patients with ALL (Abstract S102; NCT01949129). The findings in favour of TBI were pronounced enough that the study was stopped early because of significantly poorer outcomes in those patients for whom TBI was omitted. Following a mean observation time of 2.1 years, overall survival (OS) was significantly better with TBI in combination with etoposide versus chemotherapy conditioning alone (0.91 vs 0.75 years, respectively). In addition, 2-year cumulative incidence of relapse was significantly greater with chemotherapy conditioning alone compared with TBI/etoposide (0.33 vs 0.12, respectively).
“Total body irradiation remains the standard-of-care for young patients with ALL undergoing HSCT.”
Christina Peters, St. Anna Children’s Hospital, Vienna, Austria
Elias Jabbour, The University of Texas MD Anderson Cancer Center, Houston, USA, presented updated data from a small Phase I dose-escalation study which showed that the addition of the highly selective B-cell lymphoma-2 (BCL-2) inhibitor venetoclax to low-dose navitoclax (BCL-2/BCL-extra large [BCL-XL]/BCL-W inhibitor) with chemotherapy was well-tolerated and had promising efficacy in paediatric and adult patients with relapsed/refractory ALL and lymphoblastic lymphoma (Abstract S116; NCT03181126). High response rates were achieved in heavily pretreated patients, including those with prior blinatumomab, inotuzumab ozogamicin or chimeric antigen receptor (CAR)-T cell therapy. A best response of complete remission/complete remission with incomplete haematologic recovery/complete remission with incomplete platelet counts was achieved by 25 (54%) adult patients with 15 (33%) achieving undetectable minimal residual disease (MRD). Median OS was 9.7 and 6.6 months for patients with B-cell ALL and T cell-ALL, respectively. Eleven (24%) patients went on to stem cell transplant or CAR-T cell therapy. Similar efficacy was observed in paediatric patients, with the composite response (complete remission/complete remission with incomplete haematologic recovery/complete remission with incomplete platelet counts) rate of 58%, 50% undetectable MRD, and median OS of 9.7 months. Clinical follow-up, correlative biomarker analysis and expansion cohort enrolment to assess discontinuous dosing are currently underway.
Rearrangements involving PDGFRB, PDGFRA, CSF1R, ABL1 or ABL2 genes are termed ABL-class fusions – these fusions are frequent among patients with B-cell precursor ALL and T-cell ALL (T-ALL) who respond slowly to induction therapy. Anthony Moorman, Newcastle University, Newcastle, UK, presented preliminary data from the Phase III UKALL 2011 study which suggested improved outcomes for a small cohort of patients with ALL and an ABL-class fusion responding slowly to induction therapy following treatment with a tyrosine kinase inhibitor (TKI) (Abstract S117). After a median follow-up time of 2.6 years, no patients who received a TKI at first remission have yet relapsed compared with 75% of patients who did not receive this treatment.
“Adding a TKI to chemotherapy appears to improve the outcome of high-risk patients with ALL but longer follow-up is necessary.”
Anthony Moorman, Newcastle University, Newcastle, UK
T-ALL in adults is an aggressive malignancy with low long-term remission rates and high relapse and mortality rates. As a potential target for CAR-T therapy, CD7 is present on >95% of T-ALL samples and has restricted expression on the lymphoid (T cell and natural killer cell) lineage. Xinxin Wang, Gracell Biotechnologies Co. Limited, Shanghai, China, presented data from a small ongoing proof-in-principle study which demonstrated a promising early response rate with TruUCAR™ GC027, a CD7-targeted, universal CAR-T product, for patients with relapsed/refractory T-ALL (Abstract S115; Chinese Clinical Trial Register ChiCTR1900025311). Following a single infusion of GC027, 4/5 (80%) patients had robust CAR-T cell expansion and achieved a durable MRD-negative complete remission (161 days and currently ongoing) without using any biologics as part of the preconditioning therapy or bridging to HSCT.
“GC027 has demonstrated clinical efficacy and induced a deep response in patients with manageable toxicity.”
Xinxin Wang, Gracell Biotechnologies Co. Limited, Shanghai, China
In an educational session, Michel Zwaan, Princess Máxima Center, Utrecht, The Netherlands, provided an overview of novel agents in the treatment of ALL, with a particular focus on immune therapies. The treatment armamentarium for ALL is rapidly changing after decades of mainly refining existing chemotherapy and risk-group stratification. Chemotherapy induction followed by blinatumomab consolidation may be the new treatment standard in first-relapse paediatric ALL. Re-induction MRD-negative complete response (CR) rates have shown to be higher for inotuzumab ozogamicin when compared with blinatumomab in multiple relapsed/refractory ALL. As a cytoreductive strategy, it is not yet known how best to bridge to CAR-T cell therapy, and whether the efficacy of this treatment approach is reduced by prior use of immunotherapy – thus, further understanding of the optimal sequencing of targeted approaches is needed. However, further CAR-T cell development will hopefully alleviate some of the current challenges experienced with tisagenlecleucel (a medication for the treatment of B-ALL which uses adoptive cell transfer).
“Clinicians need to familiarise themselves with new toxicities and select patients with ALL for the most adequate intervention, when needed.”
Michel Zwaan, Princess Máxima Center, Utrecht, The Netherlands
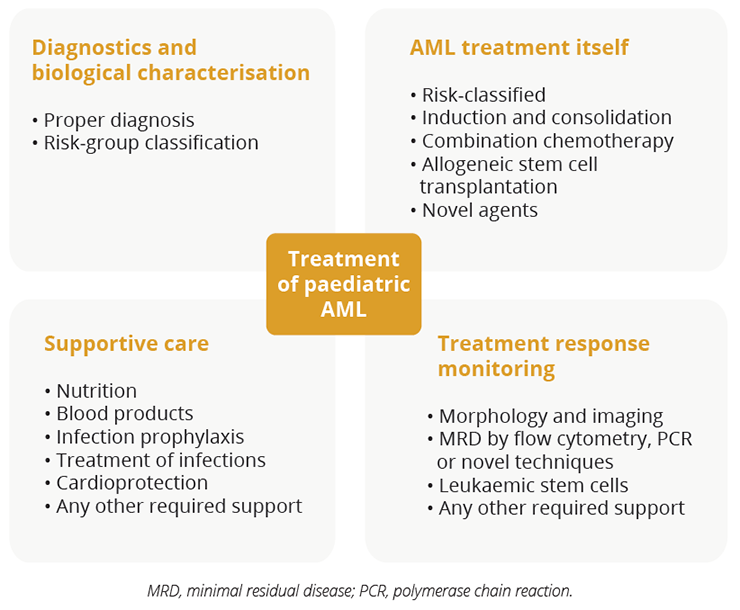
Treatment considerations for patients with paediatric AML
In an educational session, Gertjan Kaspers, Princess Máxima Center, Utrecht, The Netherlands, explained that the treatment of paediatric AML requires sophisticated diagnostics and risk-group classification. Dr Kaspers highlighted that optimal risk-group stratified treatment requires up-to-date cytogenetic and molecular characterisation along with the continued monitoring of MRD. In addition, supportive care remains crucial.
Geriatric patients with treatment-naïve AML who are ineligible for intensive chemotherapy have limited therapeutic options and poor outcomes. Amer Zeidan, Yale University and Yale Cancer Center, New Haven, USA, presented an analysis of data from the Phase III ASTRAL-1 study which suggested that both azacitidine and decitabine (hypomethylating agents) are valid treatment options for treatment-naïve geriatric patients (≥75 years) with AML who were not candidates for intensive chemotherapy (Abstract S142; NCT02348489). Patients received either guadecitabine, a next generation hypomethylating agent or a preselected treatment choice of azacitidine, decitabine or low dose cytarabine. Following a median follow up of 25.5 months, there were no significant differences in overall CR, OS or tolerability profile between azacitidine or decitabine.
“ASTRAL-1 is the largest comparison of clinical outcomes associated with azacitidine and decitabine for older treatment-naïve patients with AML ineligible for intensive chemotherapy.”
Amer Zeidan, Yale University and Yale Cancer Center, New Haven, USA

Efficacy data for azacitidine versus decitabine in the Phase III ASTRAL-1 study
Andrew H. Wei, The Alfred Hospital and Monash University, Melbourne, Australia, presented a 6-month data update from the Phase III VIALE-C study which demonstrated that the BCL-2 inhibitor venetoclax in combination with low-dose cytarabine had a clinically meaningful improvement in OS compared with low-dose cytarabine plus placebo in previously untreated older patients with AML (Abstract S136; NCT03069352). The VIALE-C study stratified patients by age (≥18 years or ≥75 years) but with comorbidities precluding intensive chemotherapy. With a median follow-up of 17.5 months, median OS was 8.4 vs 4.1 months for the venetoclax/low-dose cytarabine and placebo/low-dose cytarabine arms, respectively, representing a 30% reduction in the risk of death. Complete remission rates were 48% and 13% for the venetoclax/low-dose cytarabine and placebo/low-dose cytarabine arms, respectively. Of note, tumour lysis syndrome was only reported with venetoclax/low-dose cytarabine treatment (8/143 [5.6%] patients).
“Data from VIALE-C support venetoclax plus low-dose cytarabine as a first-line treatment option for older patients with AML, as well as those considered unfit for intensive chemotherapy.”
Andrew H. Wei, The Alfred Hospital and Monash University, Melbourne, Australia
While CAR-T cell therapy has demonstrated high success for B-cell malignancies, the translation of this therapeutic approach to AML is yet to be achieved given that AML bears heterogeneous cells that may offset killing by single CAR-based therapies, resulting in disease relapse. Leukaemic stem cells (LSCs) associated with C-type lectin-like molecule-1 (CLL-1) expression comprise a small population that plays an important role in AML disease progression and relapse, while CD33 is found on bulk AML cells in most patients with AML. Fang Liu, The General Hospital of Western Theater Command, Chengdu, China, presented data from a small Phase I first-in-human clinical study which targeted LSCs and bulk AML disease cells in patients with relapsed/refractory AML (Abstract S149). Four weeks post CAR-T cell infusion, 7 of 9 patients were MRD-negative by flow cytometry, while 2 had no response. One CD33+/CLL1- patient had no response, highlighting the importance of CLL-1 as a target for CAR-T cell therapy. Of those patients who were MRD-negative, 5 subsequently received HSCT and were successfully engrafted. Cytokine release syndrome (CRS) occurred in 8 patients, while neurotoxicity was reported in 4 patients. All episodes of CRS and neurotoxicity were resolved after treatment.
“Our study suggests that CLL-1-CD33 compound CAR-T cell therapy is a novel therapy with high efficacy and manageable toxicity in patients with relapsed/refractory AML.”
Fang Liu, The General Hospital of Western Theater Command, Chengdu, China
Closing Remarks
In response to the COVID-19 pandemic, EHA completely reimagined and reinvented its 2020 Annual Meeting to deliver EHA25 Virtual – a successful virtual congress space. This interactive virtual platform still had the aim of achieving an engaging and educational meeting experience for participants from around the world. EHA25 Virtual will no doubt stimulate new ways of thinking and ultimately translate into optimal patient care within the haematology field.
©Springer Healthcare 2020. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.