
The Annual Meeting of the European Hematology Association (EHA), now in its 25th year, took place under a new virtual format on 11–21 June 2020. Opening the meeting, EHA President John Gribben highlighted that this year’s theme was ‘Unfolding the future!” and although the COVID-19 pandemic changed the format of this year’s EHA 2020 – and renamed it to ‘EHA25 Virtual’ – the congress remained the leading meeting place for haematologists in all fields of this specialty within Europe and beyond.

John Gribben, EHA President
Professor Gribben highlighted that this year’s interactive virtual format still enabled delegates to immerse themselves in the latest news in haematology and its subspecialties. Delegates had access to clinical and translational research data of new approaches to diagnosis and treatment, including information on innovative techniques, diagnostic tools and risk-assessment strategies.
“This year’s virtual meeting will deliver haematological innovations and evidence-based knowledge of primary clinical relevance.”
John Gribben, EHA President
Lymphoma
Genetics
Hodgkin lymphoma (HL) remains a largely curative malignancy. However, the current challenge is how best to maintain treatment efficacy while minimising both short- and long-term toxicities. In addition, there is an ongoing need for reliable biomarkers of response and prognosis. The genomic landscape of HL is not well understood as the scarcity of Hodgkin-Reed-Sternberg (HRS) cells renders conventional genotyping difficult. At the same time, HRS cells release substantial amounts of circulating tumour DNA. Sven Borchmann, University of Cologne, Köln, Germany, demonstrated that HL can be comprehensively genotyped by using a liquid biopsy-based whole exome sequencing approach (Abstract S246). Several known and novel recurrently mutated genes were identified, with high mutational burden HL associated with immune escape variants (9p amplification, beta 2 microglobulin [B2M] mutations). In addition, biologically defined clusters were associated with clinical features, while a preliminary biological risk model identified high- and low-risk patients.
The genomic landscape in HL
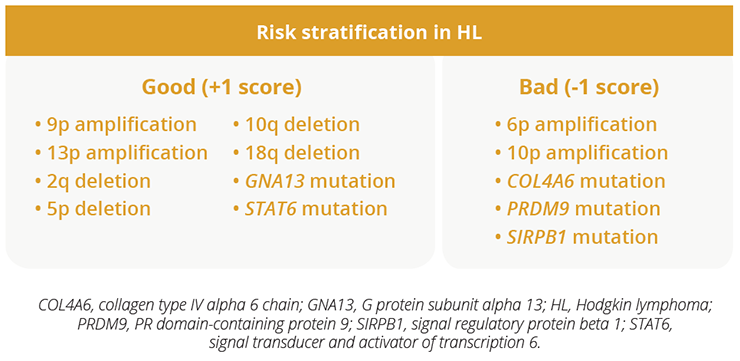
The genomic landscape in HL
“We need to understand the biological diversity of HL in order to improve treatment.”
Sven Borchmann, University of Cologne, Köln, Germany
Treatment
For decades, combined modality treatment (CMT) comprising of four cycles of chemotherapy and radiotherapy has been the standard-of-care in early-stage HL with risk factors indicating an unfavourable prognosis (‘early-stage unfavourable HL’). CMT has been efficacious regarding lymphoma control. However, the use of radiotherapy in this young patient group (median age of around 30 years at disease onset) raises concerns regarding adverse events in the long-term, such as cardiovascular disease and secondary malignancies. Peter Borchmann, University Hospital of Cologne, Köln, Germany, presented final data from the Phase III HD17 study which demonstrated that radiotherapy can be omitted from the CMT for patients that respond well to chemotherapy, as determined by fluorodeoxyglucose F-18-positron-emission tomography (PET), without loss of tumour control (Abstract S101; NCT01356680). Importantly, most patients responded well to chemotherapy alone with a 5-year overall survival (OS) rate of 98.4% versus 98.8% with CMT. Ten fatal events have occurred in the HD17 study, including two HL-related events and one treatment-related death, with the mortality of patients with early stage unfavourable HL similar to that of the healthy German population.
“PET-guided therapy reduces the proportion of patients at risk of late effects from irradiation.”
Peter Borchmann, University Hospital of Cologne, Köln, Germany
Patients with relapsed/refractory classical HL have few treatment options when they are no longer eligible for autologous stem cell transplantation (SCT) due to chemorefractory disease, comorbidities or advanced age. However, Phase II data have demonstrated antitumour activity and tolerable safety with programmed cell death protein 1 (PD-1) blockade using pembrolizumab monotherapy in this patient group. Pier Luigi Zinzani, University of Bologna, Bologna, Italy, presented data from the Phase III KEYNOTE-204 study which demonstrated that pembrolizumab was superior to the antibody-drug conjugate brentuximab vedotin in patients with relapsed/refractory classical HL (progression-free survival [PFS] of 13.2 vs 8.3 months, respectively) (Abstract LB2600; NCT02684292). In addition, this PFS benefit extended to key subgroups of patients, including those with primary refractory disease and those who had not received prior autologous SCT. Safety was consistent with the known profiles of each agent.
“Data from KEYNOTE-204 support the use of pembrolizumab monotherapy as the new standard-of-care for patients with relapsed/refractory classical HL.”
Pier Luigi Zinzani, University of Bologna, Bologna, Italy
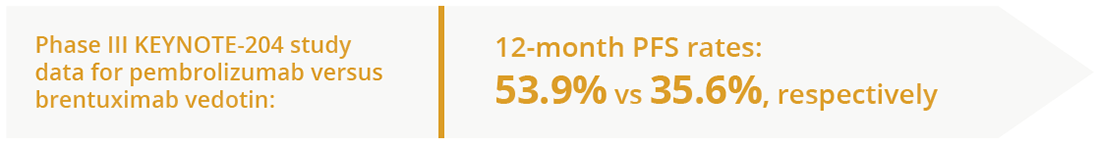
Efficacy data of the Phase III KEYNOTE-204 study
CD19 chimeric antigen receptor (CAR)-T cell therapies have shown significant activity in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), although relapses due to CD19 loss or programmed death-ligand 1 (PD-L1) upregulation remain common. Wendy Osborne, Freeman Hospital, Newcastle, UK, presented data from the Phase I Alexander study which showed that AUTO3 (a CAR-T cell therapy designed to target CD19 and CD22 simultaneously with limited duration of anti-PD1 blockade) at >150 x 106 CAR-T cells together with pembrolizumab induced complete responses (CRs) in patients with relapsed/refractory DLBCL (Abstract S240; NCT03287817). In addition, no patient experienced severe cytokine release syndrome or neurotoxicities of any grade. All CRs are currently ongoing at a dose of 150 x 106 CAR-T cells with pembrolizumab.
“Dual targeting CD19 and CD22 together with pembrolizumab pre-conditioning is a promising treatment strategy for relapsed/refractory DLBCL.”
Wendy Osborne, Freeman Hospital, Newcastle, UK
Overall response rate of 75% and CR rate of 63% at a dose of ≥150 x 106 CAR-T cells with a single dose of pembrolizumab on Day 1
Central nervous system (CNS) recurrence of DLBCL is a rare, yet devastating, event associated with poor outcomes. In addition, the identification of patients at risk and the role, method and timing of prophylactic therapy remain controversial. Matthew Wilson, Beatson West of Scotland Cancer Centre, Glasgow, UK, presented data from the first study to examine intercalating (between R-CHOP [rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone] cycles) versus end-of-R-CHOP treatment (EOT) delivery of high-dose methotrexate in patients with DLBCL (Abstract S236). Intercalated high-dose methotrexate with R-CHOP caused increased toxicity and R-CHOP delay compared with EOT delivery. Significantly more mucositis and neutropaenia were reported with intercalated high-dose methotrexate versus EOT use. R-CHOP delays were reduced if intercalated high-dose methotrexate was delivered before Day 10 of the R-CHOP cycles. No significant differences in CNS relapse, PFS, or OS were observed between the two approaches in this retrospective analysis.
“Our data support delivering high-dose methotrexate as early as possible during or following R-CHOP in patients with DLBCL.”
Matthew Wilson, Beatson West of Scotland Cancer Centre, Glasgow, UK
In an educational session, Emanuele Zucca, Oncology Institute of Southern Switzerland, Bellinzona, Switzerland, discussed the chemotherapy-free treatment of indolent lymphomas. Dr Zucca highlighted the ‘need of therapy’ definition as at least one of the following:
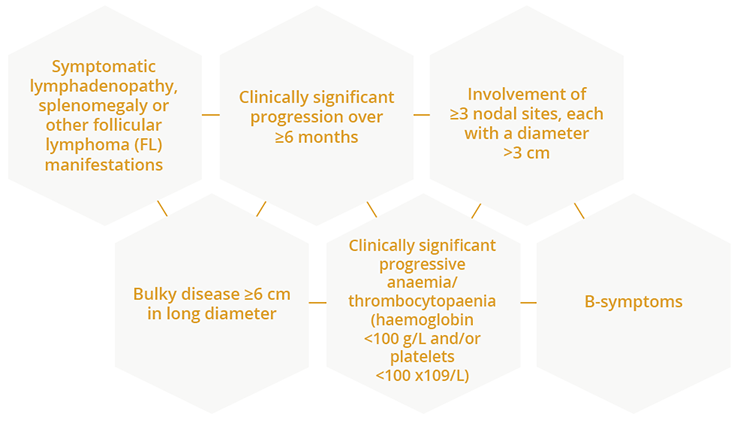
Considerations for the need of chemotherapy-free treatment of indolent lymphomas
Based on available evidence, single-agent rituximab appears to provide long-term clinical benefit in more than one third of patients. Of note, initial treatment with rituximab monotherapy does not appear to impair long-term OS. The combination of lenalidomide and rituximab, known as the R2 regimen, is a potential new option for the upfront treatment of FL.
“Chemotherapy-free treatment approaches for indolent lymphoma require further investigation, although rituximab may still be the benchmark.”
Emanuele Zucca, Oncology Institute of Southern Switzerland, Bellinzona, Switzerland
COVID-19 infection in lymphoma
Gianluca Gaidano, University of Eastern Piedmont, Novara, Italy, provided an overview of the management of patients with lymphoma during the current COVID-19 pandemic. Dr Gaidano explained that patients with cancer had poorer outcomes from COVID-19, including a higher risk of severe events (a composite endpoint defined as the percentage of patients being admitted to the intensive care unit requiring invasive ventilation, or death) compared with patients without cancer. Available data suggest patients with genitourinary and haematological malignancies have the worst survival outcome compared with other cancer types. Of note, provision of anti-cancer treatment was not associated with worse mortality. There is an unmet need for evidence-based medicine data on the management of COVID-19 in lymphoma, leaving expert opinion as the sole evidence for clinical reference. Dr Gaidano highlighted that the usual two-week interval for COVID-19 testing may not be suitable for patients with haematological diseases given the risk of virus reactivation in this patient group. Clinical data from the Hospital Clinic, Barcelona, Spain, showed that of 420 patients with chronic lymphocytic leukaemia (CLL), 4 (0.95%) were diagnosed with symptomatic COVID-19, all of whom had a mild disease course which did not necessitate the need for intensive care.
“The seemingly low prevalence of symptomatic COVID-19 in CLL needs to be interpreted with caution and warrants additional studies.”
Gianluca Gaidano, University of Eastern Piedmont, Novara, Italy
Closing Remarks
In response to the COVID-19 pandemic, EHA completely reimagined and reinvented its 2020 Annual Meeting to deliver EHA25 Virtual – a successful virtual congress space. This interactive virtual platform still had the aim of achieving an engaging and educational meeting experience for participants from around the world. EHA25 Virtual will no doubt stimulate new ways of thinking and ultimately translate into optimal patient care within the haematology field.
©Springer Healthcare 2020. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.