
Welcome to ESMO 2020

An overview of the sessions and attendees at ESMO 2020
The Annual Meeting of the European Society of Medical Oncology (ESMO) took place under a new virtual format on 19–21 September 2020, in collaboration with the European Oncology Nursing Society (EONS). Even during a pandemic, efforts to fight cancer do not stop and leading oncology experts from all corners of the world were reunited as one oncology community to share the latest advances in the field at the ESMO Virtual Congress 2020. Indeed, this year’s tagline of ‘Bringing innovation to cancer patients’ was particularly timely in the current era where there is an urgent need to put the latest real-world evidence into clinical practice. The ESMO Virtual Congress 2020 programme provided the first announcements of practice-changing data and ground-breaking translational cancer research that delegates have come to expect from the Society’s annual Congress.

“The ESMO Virtual Congress 2020 is certainly something new and different, but its essence is there: sharing the latest research results to bring innovations to patients with cancer. ”
Solange Peters, ESMO and Congress 2020 President
Breast cancer
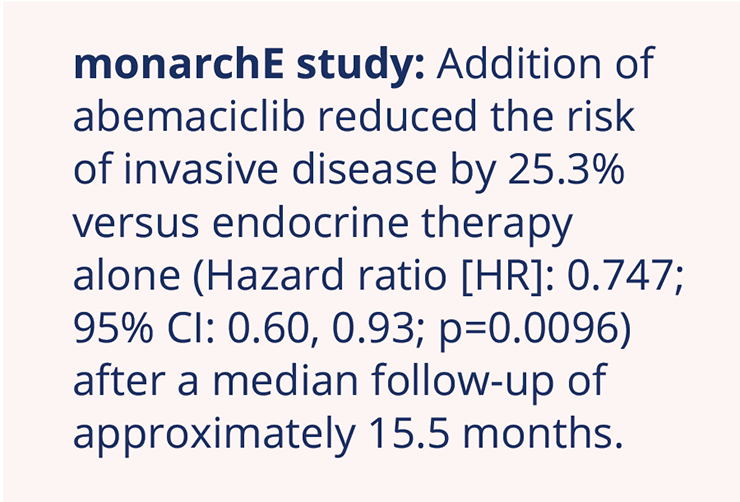
Key findings of the Phase III monarchE study of abemaciclib vs. endocrine therapy alone
Based on the overall survival (OS) advantage that the cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, abemaciclib, has shown in combination with fulvestrant in the metastatic breast cancer setting, investigators evaluated adjuvant abemaciclib in combination with endocrine therapy. Stephen Johnston, The Royal Marsden NHS Foundation Trust, London, UK, presented data from the Phase III monarchE study which showed that the addition of abemaciclib to endocrine therapy led to a significant reduction in the risk of invasive disease versus endocrine therapy alone in patients with high-risk early hormone receptor (HR)-positive, human epidermal growth factor receptor 2 HER2– breast cancer (NCT03155997; LBA5_PR).
Two-year invasive disease-free survival (iDFS) rates were 92.2% in the abemaciclib arm versus 88.7% in the endocrine-alone arm, reflecting an absolute between-group improvement of 3.5%. Further results demonstrated that the addition of abemaciclib to endocrine therapy also had a significant impact on distant relapse-free survival across all prespecified subgroups, reducing the risk of distant recurrence by 28.3% (HR: 0.717; 95% CI: 0.56, 0.92; p=0.0085).
“Abemaciclib is the first CDK4/6 inhibitor to show a significant improvement in iDFS when combined with endocrine therapy”
Stephen Johnston, The Royal Marsden NHS Foundation Trust, London, UK
Discussant George Sledge Jr, Stanford University, California, USA, highlighted that some key unanswered questions remain, such as whether improved DFS would translate into improved OS and whether CDK4/6 inhibition could reduce late disease recurrence.
Fulvestrant and the CDK4/6 inhibitor, palbociclib, have previously been proven to be a standard-of-care in the treatment of patients with HR+, HER2– metastatic breast cancer who had failed on prior treatment based on data from the PALOMA-3 study, which demonstrated a significant improvement in OS compared with fulvestrant alone. However, PALOMA-3 did not include patients with endocrine-sensitive disease. Joan Albanell, Hospital del Mar, Barcelona, Spain, presented data from the Phase II FLIPPER study (GEICAM/2014-12; NCT02690480) to show that the use of this treatment combination as first-line (1L) therapy improved 1-year progression-free survival (PFS) compared with fulvestrant and placebo alone in postmenopausal women with HR+, HER2–, endocrine-sensitive, metastatic breast cancer (LBA19). The doublet regimen led to improvements in median PFS, objective response rate (ORR), and clinical benefit rate (CBR) compared with fulvestrant alone.
Key findings from the Phase II FLIPPER study
PFS at 1 year was 83.5% with palbociclib and fulvestrant versus 71.9% with fulvestrant and placebo (HR: 0.55; 80% CI: 0.36, 0.83; p=0.064) after a median follow-up of 28.6 months
Median PFS was 31.8 months with palbociclib and fulvestrant and 22 months with fulvestrant alone (adjusted HR: 0.52; 80% CI: 0.39, 0.68; p=0.002)
PFS data in the CheckMate 649 study
OS data were not yet mature at the time of the data cut-off and therefore not reported. While approximately 20% of patients discontinued treatment with palbociclib and fulvestrant, Dr Albanell described the tolerability profile of this combination as being manageable.
“In exploratory trials such as the FLIPPER study, the results are hypothesis-generating and have to be interpreted as such.”
Joan Albanell, Hospital del Mar, Barcelona, Spain
A number of presentations at this year’s meeting focused on health-related quality of life (HRQoL) changes with the treatment of locally advanced or metastatic breast cancer. Veliparib, a poly (ADP-ribose) polymerase 1/2 inhibitor, has previously demonstrated survival benefits in the Phase III BROCADE 3 study when combined with paclitaxel/carboplatin in patients with HER2– metastatic or locally advanced unresectable germline BRCA-associated breast cancer, in which the combination significantly prolonged PFS over paclitaxel/carboplatin alone (NCT02163694). Michael Friedlander, The Prince of Wales Hospital, Randwick, Australia, presented additional data from this study to show that addition of veliparib to paclitaxel/carboplatin has no detrimental effect on QoL and may be beneficial in some areas of functioning and symptom experience (Abstract 274O).
Median time to symptom worsening was significantly longer (p<0.05) for veliparib plus paclitaxel/carboplatin versus paclitaxel/carboplatin alone for:
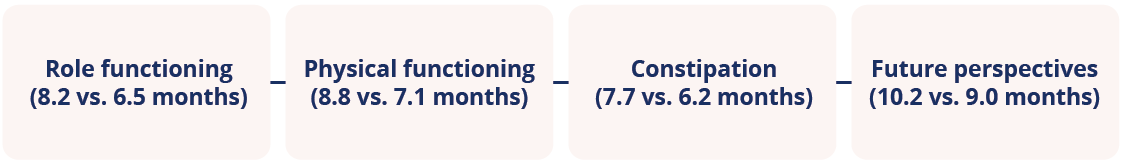
Median time to symptom worsening in patients receiving paclitaxel/carboplatin versus paclitaxel/carboplatin alone in the BROCADE study
Patients with HER2+ metastatic breast cancer, particularly those with brain metastases, have limited treatment options and an increased likelihood to report deterioration in HRQoL. Volkmar Mueller, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, presented data on the impact of the small molecule inhibitor of HER2, tucatinib, on HRQoL in patients with HER2+ metastatic breast cancer with and without brain metastases from the HER2CLIMB study (Abstract 275O; NCT02614794). While the addition of tucatinib to trastuzumab and capecitabine resulted in statistically significant and clinically meaningful improvement in PFS and OS, QoL in those patients treated with this regimen was maintained throughout the study period for a longer duration compared with those patients receiving trastuzumab and capecitabine only.
“No clinically meaningful differences in HRQoL were observed between tucatinib–trastuzumab–capecitabine and trastuzumab–capecitabine treatment arms in HER2+ metastatic breast cancer with and without brain metastases.”
Volkmar Mueller, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Patient-reported QoL data have been presented separately for each of the Phase III MONALEESA studies (2 [NCT01958021], 3 [NCT02422615], and 7 [NCT02278120]), which investigated the efficacy and safety of ribociclib in combination with different endocrine therapies as 1L or second-line (2L) treatment for HR+/HER2– advanced breast cancer. Peter A. Fasching, Universitätsklinikum Erlangen, Erlangen, Germany, presented a pooled analysis of QoL outputs from these studies, which demonstrated that ribociclib delayed deterioration in QoL in those patients who received 1L endocrine therapy across the MONALEESA studies (Abstract 276O). Time-to-definitive deterioration (TTDD) ≥10% for global health status (GHS) and emotional functioning was delayed with ribociclib:
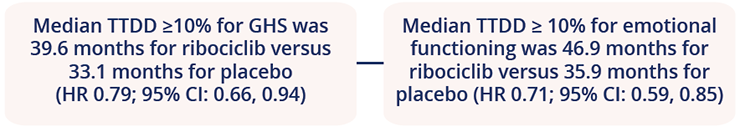
A pooled analysis of patient-reported QoL data in the MONALEESA studies
“This large, robust analysis demonstrated favourable QoL results with the addition of ribociclib to endocrine therapy in patients with HR+/ HER2– advanced breast cancer.”
Peter A. Fasching, Universitätsklinikum Erlangen, Erlangen, Germany
Closing Remarks
In summary, ESMO 2020 was a successful virtual meeting with delegates having had the chance to remotely access new data, clinical experiences, patient perspectives, and best practices that will hopefully stimulate new ways of thinking and ultimately translate into optimal patient care within the oncology field. In addition, with the ongoing COVID-19 pandemic and the shift to distance learning and virtual meetings, ESMO acknowledged this opportunity to further extend their educational reach by providing accessible virtual content to physicians located in regions far removed from the cancer conference circuit.
©Springer Healthcare 2020. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.