
Welcome to ESMO 2020

An overview of the sessions and attendees at ESMO 2020
The Annual Meeting of the European Society of Medical Oncology (ESMO) took place under a new virtual format on 19–21 September 2020, in collaboration with the European Oncology Nursing Society (EONS). Even during a pandemic, efforts to fight cancer do not stop and leading oncology experts from all corners of the world were reunited as one oncology community to share the latest advances in the field at the ESMO Virtual Congress 2020. Indeed, this year’s tagline of ‘Bringing innovation to cancer patients’ was particularly timely in the current era where there is an urgent need to put the latest real-world evidence into clinical practice. The ESMO Virtual Congress 2020 programme provided the first announcements of practice-changing data and ground-breaking translational cancer research that delegates have come to expect from the Society’s annual Congress.

“The ESMO Virtual Congress 2020 is certainly something new and different, but its essence is there: sharing the latest research results to bring innovations to patients with cancer. ”
Solange Peters, ESMO and Congress 2020 President
Lung Cancer

Key findings of the ADAURA study of patients with resected EGFRm NSCLC
Central nervous system (CNS) relapse is common in non-small cell lung cancer (NSCLC), and is a poor prognostic factor. In the resected EGFR-sensitising mutation (EGFR) NSCLC setting, the impact of treatment on sites of recurrence, including the CNS, is a key consideration. Masahiro Tsuboi, Cancer Center Hospital East, Kashiwa, Japan, presented an exploratory analysis of data from the Phase III ADAURA study (NCT02511106) which showed the risk of developing disease recurrence in the CNS to be significantly lower and clinically meaningful in patients with completely resected stage IB–IIIA EGFRm NSCLC treated with the third-generation EGFR inhibitor osimertinib compared with placebo (LBA1). At a median follow-up of 22 months, 11% (37/339) of patients in the osimertinib arm and 46% (159/343) of those in the placebo arm experienced a disease-free survival (DFS) event characterised by disease recurrence or death. The presence of distant disease was represented by disease recurrence in 38% (14/37) and 61% (96/157) of those receiving osimertinib and placebo, respectively.
“The reduced risk of local and distant recurrence and improved CNS DFS reinforce adjuvant osimertinib as a highly effective, practice-changing treatment for patients with stage IB/II/IIIA EGFRm NSCLC following complete tumour resection.”
Masahiro Tsuboi, Cancer Center Hospital East, Kashiwa, Japan
Results for overall survival (OS), a secondary study endpoint, were immature at the time of this analysis. Discussant Johan Vansteenkiste, University Hospitals Leuven, Campus Gasthuisberg, Belgium, suggested that minimal residual disease may ultimately play a crucial role in the selection of patients eligible to receive the drug.
“While it appears there may be superior CNS control with osimertinib, DFS might not translate to better cure rates.”
Discussant Johan Vansteenkiste, University Hospitals Leuven, Campus Gasthuisberg, Belgium
Benjamin Solomon, Peter MacCallum Cancer Centre, Melbourne, Australia, presented data from a planned interim analysis of the Phase III CROWN study (NCT03052608) which compared lorlatinib with crizotinib in the first-line (1L) treatment of patients with advanced ALK-positive (ALK+) NSCLC (LBA2). Dr Solomon highlighted that lorlatinib significantly improved progression-free survival (PFS) compared with crizotinib and support the use of lorlatinib as a 1L treatment for patients with ALK rearrangement-positive NSCLC.
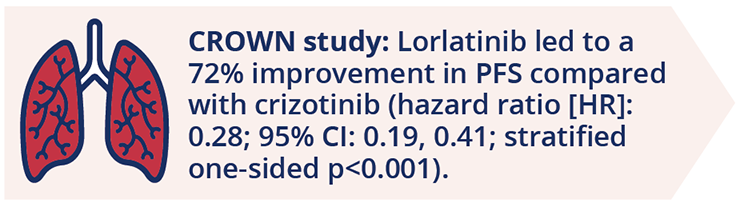
PFS improvement with lorlatinib in patients with advanced ALK-positive (ALK+) NSCLC
Lorlatinib was also associated with numerical improvements in best overall response (BOR). Three-quarters (n=113 [76%]) of patients receiving lorlatinib achieved a complete response (CR; n=4) or a partial response (PR; n=109). This was compared with a 58% BOR rate for crizotinib, with no CRs and 85 PRs. Of note, while the incidence of grade 3/4 adverse events (AEs) was higher with lorlatinib (72.5%) than crizotinib (55.6%), fewer patients receiving lorlatinib, compared with crizotinib, experienced AEs leading to treatment discontinuation (6.7% versus 9.2%, respectively).
David Hong, The University of Texas MD Anderson Cancer Center, Houston, USA, presented data to show that the investigational drug sotorasib (AMG 510) demonstrated antitumour activity in patients with advanced NSCLC that harbour the KRAS p.G12C mutation, an aberration for which no targeted treatment has yet been approved (Abstract 1257O). In a small subgroup of the Phase I CodeBreaK100 study that included 59 heavily pre-treated patients (median of two prior lines of systemic anticancer therapy, consistent with their tumour type and stage of disease) with KRAS p.G12C-positive NSCLC (NCT03600883), researchers found that 32.2% experienced a confirmed objective response, while 88.1% achieved disease control.
“Sotorasib is a first-in-class small molecule that selectively and irreversibly targets KRAS p.G12C.”
David Hong, The University of Texas MD Anderson Cancer Center, Houston, USA
Data from another Phase I study, CHRYSALIS (NCT02609776), demonstrated high response rates with the combination of the EGFR-MET bispecific antibody, amivantamab, and the third-generation tyrosine kinase inhibitor (TKI) lazertinib in treatment-naïve and osimertinib-resistant patients with advanced EGFRm NSCLC (Abstract 1258O). At a median follow-up of 7 months in treatment-naïve patients, the objective response rate (ORR) and the clinical benefit rate (CBR) was 100%. At a median follow-up of 4 months in osimertinib-resistant, chemotherapy-naïve patients, the ORR and CBR were 36% and 60%, respectively. Based on these data, new studies with amivantamab and lazertinib have been initiated, including the Phase III MARIPOSA and Phase II CHRYSALIS-2 studies in the frontline and chemotherapy-relapsed settings, respectively.
Dual inhibition of both VEGFR and EGFR with the combination of the multi-targeted tyrosine kinase inhibitor (TKI) apatinib and gefitinib in the 1L treatment of patients with advanced EGFRm NSCLC demonstrated superior PFS, according to data from the Phase III ACTIVE study (CTONG170; NCT02824458) presented by Li Zhang, Sun Yat-sen University Cancer Center, Guangzhou, China (LBA50). This study was based on early preclinical findings which demonstrated that dual blockade delayed the emergence of resistant tumours.

Key efficacy data in the Phase III ACTIVE study
Stratified PFS data showed a similar PFS benefit for patients with Ex19del (HR: 0.67; 95% CI: 0.45, 0.99) and those with L858R mutations (HR: 0.72; 95% CI: 0.48, 1.09). No unexpected safety signals were identified beyond the established safety profile of each single agent. Dr Zhang explained that this dual oral regimen will definitely provide a more convenient treatment for patients who require long-term treatment administration.
“Apatinib in combination with gefitinib is expected to become a new 1L treatment option for EGFR-m NSCLC patients.“
Li Zhang, Sun Yat-sen University Cancer Center, Guangzhou, China
Post-operative radiotherapy (PORT) was linked with a non-statistically significant increase in DFS in patients with completely resected stage IIIA-N2 NSCLC and thus cannot be recommended as a standard-of-care in this population, according to data from the Phase III LungART study (NCT00410683) presented by Cécile Le Pechoux, Institut de Cancérologie Gustave Roussy, Villejuif, France (LBA3_PR). Dr Le Pechoux explained that the median DFS in the control arm where patients did not receive PORT was 22.8 months compared with 30.5 months in those who received PORT. The 3-year DFS rates were estimated to be 43.8% and 47.1% in the control arm and PORT arm, respectively. OS rates at 3 years were 68.5% in the control arm and 66.5% in the PORT arm.
“The use of PORT was associated with a 15% increase in DFS that was determined to be non-statistically significant, with no difference in 3-year OS.”
Cécile Le Pechoux, Institut de Cancérologie Gustave Roussy, Villejuif, France
Closing Remarks
In summary, ESMO 2020 was a successful virtual meeting with delegates having had the chance to remotely access new data, clinical experiences, patient perspectives, and best practices that will hopefully stimulate new ways of thinking and ultimately translate into optimal patient care within the oncology field. In addition, with the ongoing COVID-19 pandemic and the shift to distance learning and virtual meetings, ESMO acknowledged this opportunity to further extend their educational reach by providing accessible virtual content to physicians located in regions far removed from the cancer conference circuit.
©Springer Healthcare 2020. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.