
Welcome to ESMO 2021
The first two days of the ESMO Congress 2021 have showcased two positive breast cancer trials in the early triple-negative (TNBC) and HER2-positive metastatic settings, as well as research in patients with hormone receptor-positive/HER2-negative advanced disease.
Virtual plenary debate: The KEYNOTE-522 trial
The breast cancer conference track opened with the discussion of the KEYNOTE-522 trial (NCT03036488) findings that were previously reported this year at the ESMO Virtual Plenary by Peter Schmid, from Barts Cancer Institute at Queen Mary University in London, UK.
The phase 3 study demonstrated that patients with early-stage TNBC achieve significantly better rates of pathological complete response (pCR) and event-free survival (EFS) with a regimen of neoadjuvant pembrolizumab plus platinum-based chemotherapy followed by pembrolizumab after surgery than with neoadjuvant chemotherapy plus placebo.
Invited discussant Lisa Carey, from the University of North Carolina, Chapel Hill, USA, questioned which patients benefit from the addition of immune checkpoint immunotherapy (ICI) and whether it was necessary in patients with pCR who did well in both the pembrolizumab and placebo treatment groups, with EFS rates of 94.4% and 92.5%, respectively.
Schmid responded that benefits were seen across all patient subgroups, including those with or without positive lymph nodes or PD-L1-positive tumours. He emphasized that pCR is predictive of better outcome regardless of pembrolizumab receipt but that pembrolizumab-treated patients with pCR had better EFS than their placebo-treated counterparts.
For patients with residual disease, EFS was better with pembrolizumab than placebo, suggesting that lack of pCR may not rule out an EFS benefit. “That’s important when we look at the design of future neoadjuvant trials”, he remarked, adding that work continues to determine the “important question” of how to “reliably identify” patients who will achieve pCR with chemotherapy alone.

Schmid commented that neither capecitabine, nor olaparib were used within the KEYNOTE-522 trial population, but that safety data from other studies support their combination with pembrolizumab and that individual decision-making may permit adjuvant use of capecitabine in patients without pCR or olaparib in those with a germline BRCA mutation.
T-DXd as second-line treatment for HER2-positive advanced breast cancer
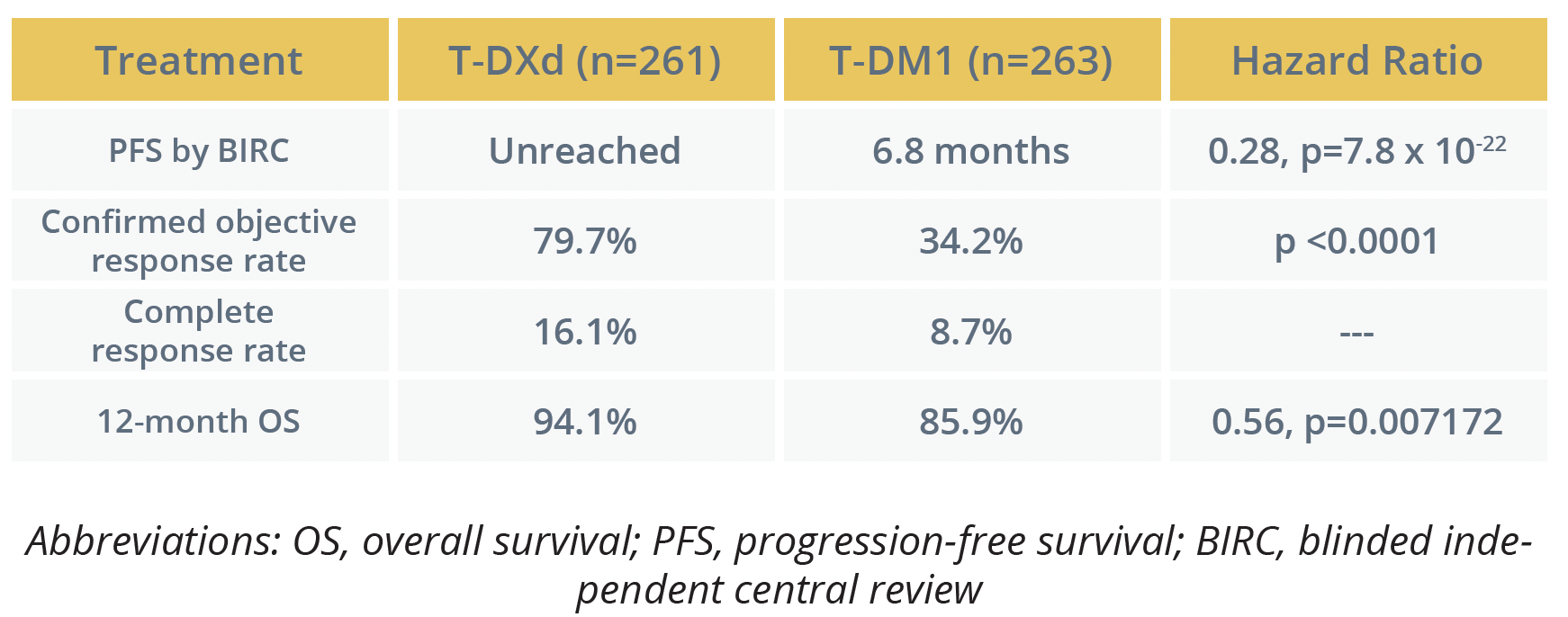
The results of the DESTINY-Breast03 trial (NCT03529110), presented in the Presidential symposium, demonstrate a highly significant progression-free survival (PFS) benefit for patients with HER2-positive unresectable or metastatic breast cancer treated with the antibody–drug conjugate trastuzumab deruxtecan (T-DXd) compared with trastuzumab emtansine (T-DM1).
After a median 16.2 months of follow-up, the primary endpoint of median PFS by blinded independent central review was unreached in the patients randomly assigned to receive T-DXd 5.4 mg/kg every 3 weeks versus 6.8 months for those given T-DM1 3.6 mg/kg every 3 weeks and followed up for a median of 15.3 months, with 12-month rates of 75.8% and 34.1%, respectively.
Presenting investigator Javier Cortés, from Vall d’Hebron Institute of Oncology in Barcelona, Spain, pointed to the “clear and early separation of the curves” and the hazard ratio of 0.28 with a “highly clinically meaningful and statistically significant” p value of 7.8 x 10-22.
There was a consistent PFS benefit of T-DXd across all predefined patient subgroups, including
hormone receptor status
prior use of pertuzumab
number of lines of therapy
presence of visceral metastases, and
presence of brain metastases
The key secondary endpoint of OS has not yet reached statistical significance, “likely due to immature follow-up”, the presenter observed.
Response rates and OS results
He noted that T-DXd patients received a median treatment duration of 14.3 months versus 6.9 months for those given T-DM1, but the groups had similar rates of treatment-emergent adverse events (TEAEs), with grade 3 and more severe events occurring in 45.1% and 39.8%, respectively, and no fatal TEAEs.
Of note, 12.8% of patients discontinued T-DXd because of TEAEs, most commonly interstitial lung disease (ILD)/pneumonitis (8.2%), while 5.0% of patients discontinued T-DM1, most commonly attributed to thrombocytopenia (2.7%).
T-DXd-related ILD/pneumonitis was of concern in the phase 2 DESTINY-Breast01 trial, but Cortés reported that any-grade events occurred in 10.5% with just two cases of grade 3 and none at grade 4–5. Left ventricular ejection fraction decreases occurred in 2.7% of T-DXd-treated patients, all at grade 1 or 2.
"These data support T-DXd becoming the standard of care for second-line HER2-positive metastatic breast cancer”
Javier Cortés, Barcelona, Spain
Session discussant Shanu Modi, from Memorial Sloan Kettering Cancer Center in New York, USA, described the PFS curve and associated hazard ratio (HR) for DESTINY-Breast03 as “absolutely startling” and “exceptional” when compared with prior trial findings for T-DM1.
She highlighted that approximately a fifth of the patients had stable untreated brain metastases and T-DXd was associated with a threefold increase in PFS compared with T-DM1, “a very clinically important difference to highlight between these two agents”.
Modi said that the T-DXd safety profile was “reassuring” and hypothesized that the greater awareness of lung toxicity with the antibody–drug conjugate following updates to management guidelines may have reduced adverse events, implying that “vigilance and early intervention is absolutely mandatory to deliver T-DXd therapy safely”.
Metastatic breast cancer options continue to expand
The phase 3 PALOMA-4 (NCT02297438) results confirm that Asian postmenopausal women with oestrogen receptor-positive, HER2-negative advanced breast cancer derive a significant PFS benefit from the use of the CDK4/6 inhibitor palbociclib alongside first-line letrozole.
Binghe Xu, from the National Cancer Center/Cancer Hospital in Beijing, China, said the median PFS of 21.5 months for the combination versus 13.9 months for letrozole plus placebo gave a significant HR of 0.677, which is “consistent with findings from the PALOMA-2 study of mostly White patients.”
Finding no new safety concerns, he summarised that the findings support the combination as first-line therapy in this population of postmenopausal Asian women.
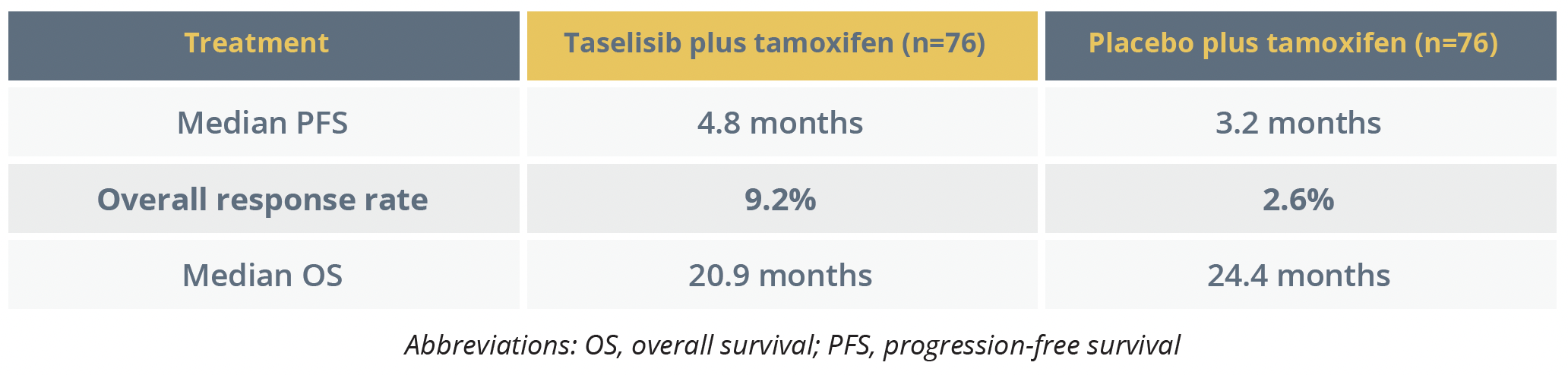
Mafalda Oliveira, from Vall d’Hebron University Hospital in Barcelona, Spain, reported the findings from the phase 2 POSEIDON trial (NCT02301988) assessing the impact of combining tamoxifen with the PI3K inhibitor taselisib in patients with hormone receptor-positive, HER2-negative metastatic breast cancer.
After a median 26.4 months, median PFS was significantly higher for the patients given taselisib plus tamoxifen than those given placebo plus tamoxifen with an HR of 0.63, accompanied by a numerically higher overall response rate.
However, OS was comparable in the treatment arms, perhaps “hampered by the cross-over design of the trial”, Oliveira remarked, and the toxicity profile of diarrhoea, hyperglycaemia and mucositis meant there was “poor” tolerability of the taselisib plus tamoxifen regimen.
Treatment outcomes
"Combining endocrine treatment and PI3K-AKT pathway inhibition using biomarkers and drugs with a better safety profile warrants further study"
Mafalda Oliveira, Barcelona, Spain
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.

