
Welcome to ESMO 2021
Sunday’s metastatic breast cancer Proffered Paper session at the ESMO Congress 2021 gave positive results for patients with HER2-positive, hormone receptor-positive, HER2-negative, and triple-negative (TNBC) forms of the disease.
TULIP trial findings blossom for SYD985
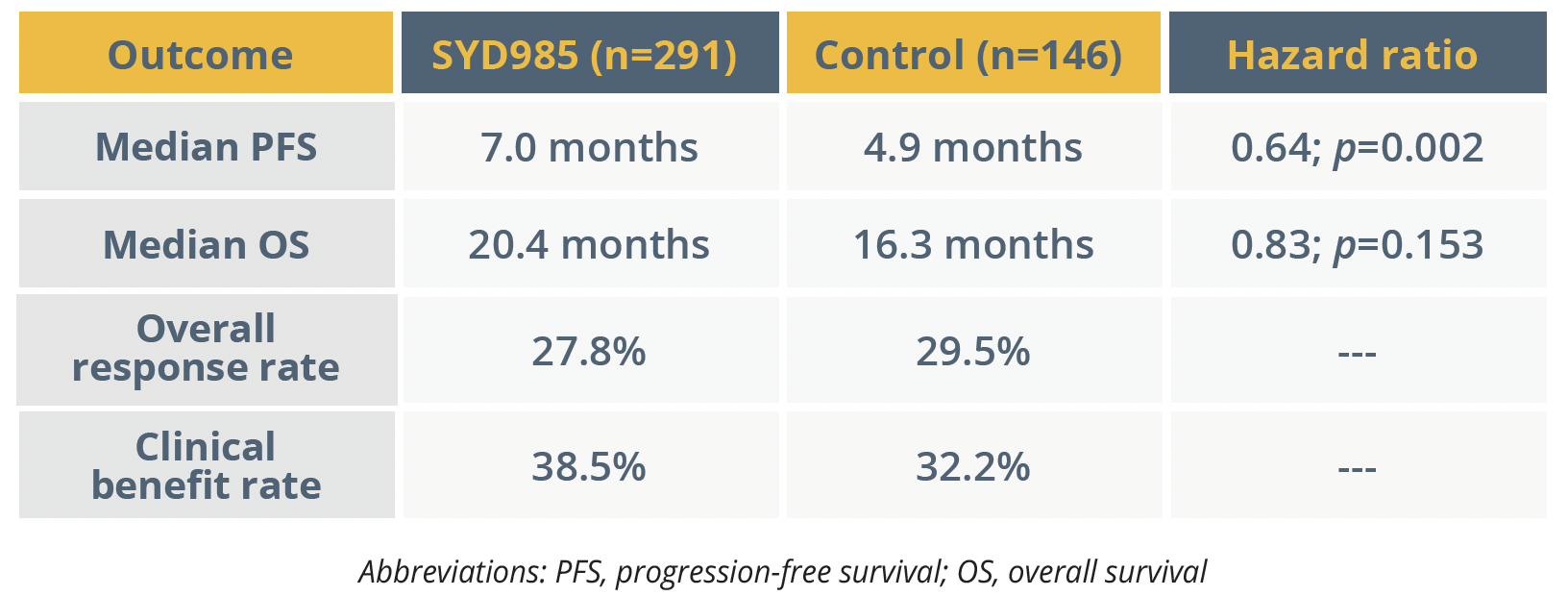
Cristina Saura, from Vall d’Hebron Institute of Oncology in Barcelona, Spain, reported the TULIP (NCT03262935) trial’s primary outcome of progression-free survival (PFS) for SYD984 (trastuzumab duocarmazine) versus a physician’s choice of treatment.
The phase 3 trial was performed in 437 patients with HER2-positive locally advanced or metastatic breast cancer, with or without treated brain metastasis, who had previously received at least two regimens or trastuzumab emtansine for metastatic disease.
The HER2-targeted antibody–drug conjugate regimen, with a cleavable linker-duocarmycin payload, was given in 21-day cycles at 1.2 mg/kg, while the control regimens consisted of lapatinib plus capecitabine, or trastuzumab plus capecitabine, vinorelbine or eribulin. PFS by central review demonstrated a significant improvement in outcomes for patients given SYD985 and the first overall survival (OS) assessment showed a trend towards a longer duration.
Outcomes for SYD985 treatment versus control
Turning to the adverse events (AEs) of special interest with SYD985, Saura said any-grade eye toxicity occurred in 78.1% of treated patients (vs 29.2% of controls) and grade 3 or more severe eye toxicity in 21.2% of patients. Despite the trial’s risk mitigation strategy, treatment modifications or discontinuation due to eye toxicity was required in around a fifth of patients given SYD985.
In addition, interstitial lung disease (ILD) and pneumonitis occurred in 7.6% of patients given the antibody–drug conjugate (vs 0.0% of controls), with grade 3 or worse AEs of this type in 2.4%. Treatment-related fatalities included two cases of pneumonitis and single cases of respiratory failure and pneumonia.
“SYD985 can provide a new treatment option for patients with pre-treated locally advanced or metastatic HER2-positive metastatic breast cancer”
Cristina Saura, Barcelona, Spain
Session discussant and chair Barbara Pistilli, from Institut Gustave Roussy in Villejuif, France, commented that “with the results of DESTINY-BREAST03 the optimal sequencing of HER2-targeting therapy is likely to change”.
She emphasized that further research will be required to assess whether SYD985 is active in patients who progress on SYD985 and to identify the optimal upfront antibody–drug conjugate for each individual.
MONALEESA-2 demonstrates 6-year OS benefit for first-line ribociclib plus letrozole
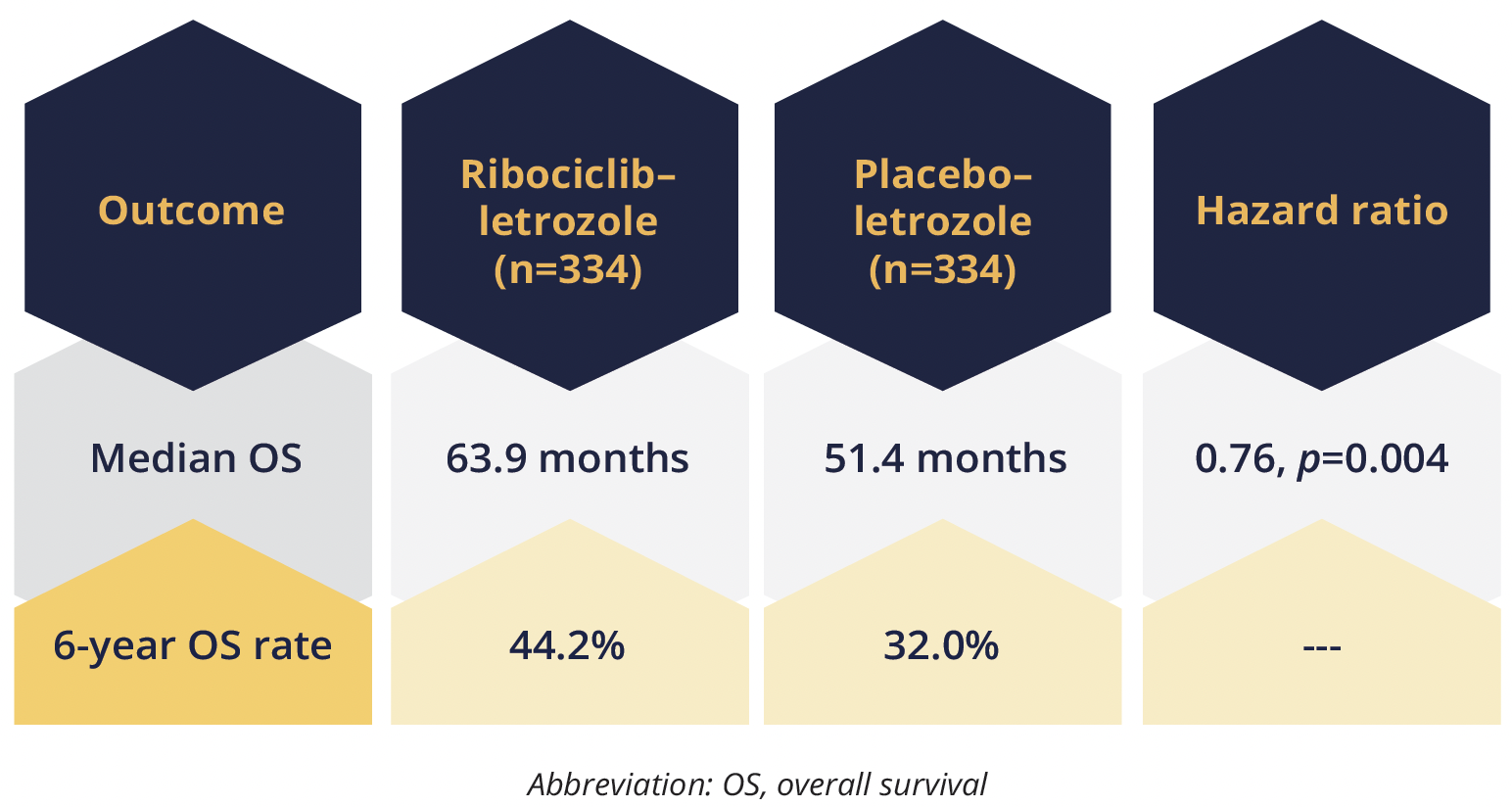
The addition of ribociclib 600 mg/day (3 weeks on, 1 week off) to first-line letrozole 2.5 mg/day significantly extends OS for patients with hormone receptor-positive, HER2-negative advanced breast cancer, the updated findings for the phase 3 MONALEESA-2 trial (NCT01958021) show after a median 80 months of follow-up. The difference in the median OS rate between the treatment groups increased over time, from 5.7% at 4 years to 12.2% at 6 years, with “consistent” benefit across patient subgroups, reported Gabriel Hortobagyi, from The University of Texas MD Anderson Cancer in Houston, USA.
The OS gain occurred despite patients given ribociclib being less likely to have subsequent CDK4/6 inhibitor use after discontinuing their study treatment (21.7 vs 34.4%), he noted.
Outcomes for the addition of ribociclib to letrozole versus letrozole alone
Patients given ribociclib also had a longer median time until chemotherapy than their control counterparts (50.6 vs 38.9 months, HR=0.74). There were no new safety signals and grade 3–4 AEs of special interest with ribociclib were reported for neutropenia (63.8 vs 1.2% with placebo), hepatobiliary toxicity (14.4 vs 4.8%), prolonged QT interval (4.5 vs 2.1%) and ILD/pneumonitis (0.6 vs 0.0%).
“Ribociclib combined with endocrine therapy is the only first-line treatment with OS benefit and should therefore be considered as the preferred treatment option for [hormone receptor]+/HER2- advanced breast cancer”
Gabriel Hortobagyi, Houston, USA
“The MONALEESA trials with ribociclib demonstrate a consistent overall survival benefit regardless of endocrine therapy partner, line of therapy, or menopausal status”, he added.
Session discussant Gonzalo Gomes-Abuin, from Hospital Alemán in Buenos Aires, Argentina, congratulated everyone involved with the trial, noting that “MONALEESA-2 is the first study to break the >5-year OS barrier in [hormone receptor]+HER2- postmenopausal metastatic breast cancer”.
KEYNOTE-355 update confirms pembrolizumab–chemotherapy efficacy
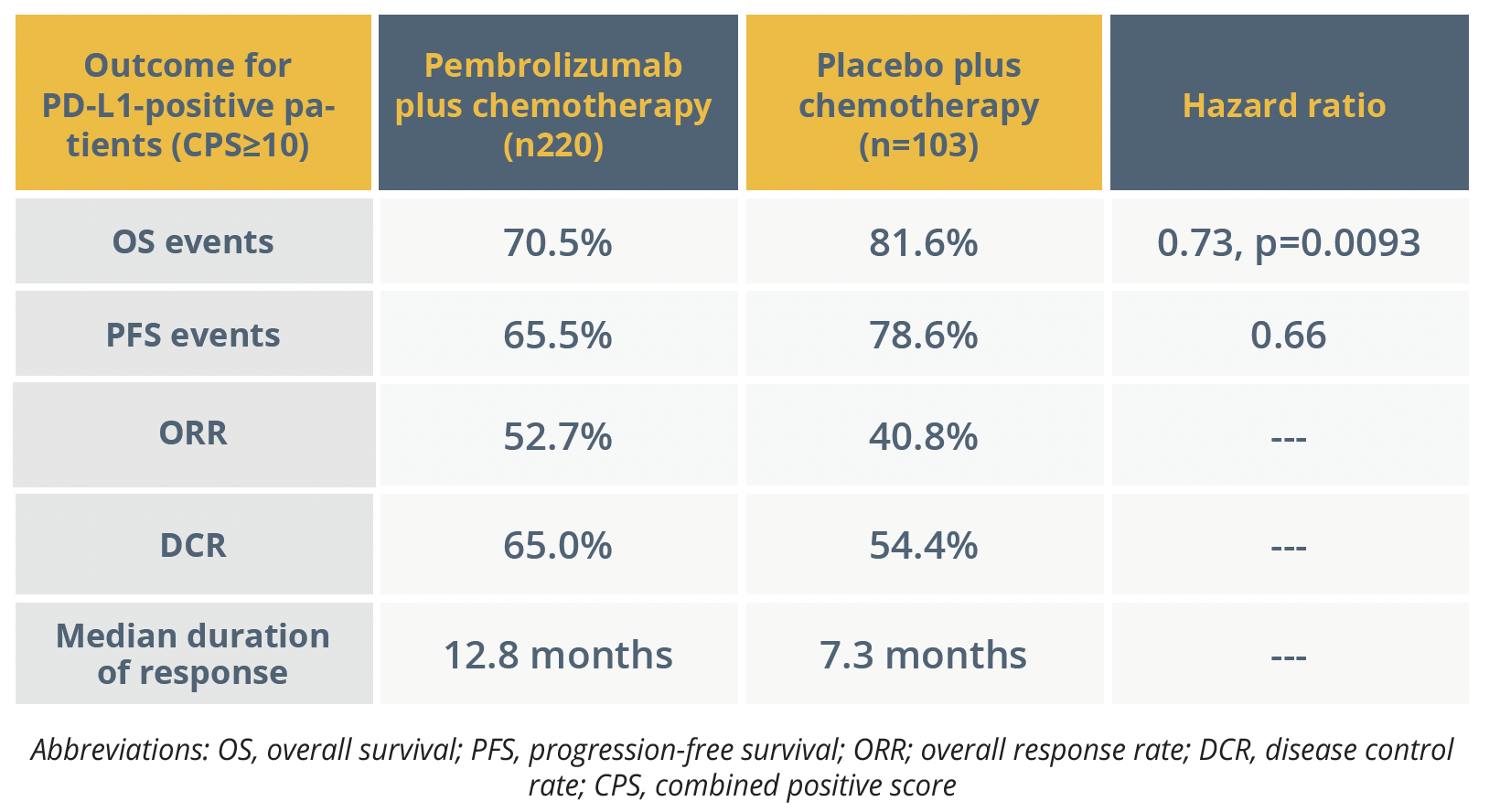
Final results were presented for the KEYNOTE-355 trial (NCT02819518) at the session, by Hope Rugo, from the University of California San Francisco in the USA.
“These results support pembrolizumab in combination with chemotherapy as a new standard-of-care treatment regimen for patients with locally recurrent unresectable or metastatic TNBC whose tumours express PD-L1 (CPS [combined positive score] ≥10)”,
Hope Rugo, San Francisco, USA
OS was significantly longer for the patients randomly assigned to receive pembrolizumab 200 mg every 3 weeks plus a chemotherapy regimen of nab-paclitaxel, paclitaxel, or gemcitabine plus carboplatin, compared with those given placebo plus chemotherapy, at a median of 23.0 versus 16.1 months.
Outcome data for pembrolizumab plus chemotherapy versus chemotherapy alone
Treatment-related AEs at grade 3–5 occurred in 68.1% of the pembrolizumab arm and 66.9% of controls, with drug discontinuation due to AEs reported in 18.3% and 11.0%, respectively. Immune-mediated AEs at grade 3–5 occurred in a corresponding 5.3% and 0.0% of the groups, with 2.8% and 0.0% discontinuing treatment because of these. “Safety was consistent with the known profiles of reach regimen, with no new safety concerns”, commented Rugo.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.
