
Welcome to ESMO 2021
The final day of sessions on genitourinary cancer heralded some practice-changing research with abiraterone in prostate cancer and important data on the potential benefits of tyrosine kinase inhibitor breaks for people with advanced renal cell cancer.
Practice-changing results for abiraterone plus ADT in metastatic prostate cancer
Adding abiraterone acetate plus prednisolone, with or without enzalutamide, to androgen deprivation therapy (ADT) significantly improves survival outcomes for men with high-risk non-metastatic prostate cancer, indicates a combined analysis of data from the STAMPEDE trial (NCT00268476).
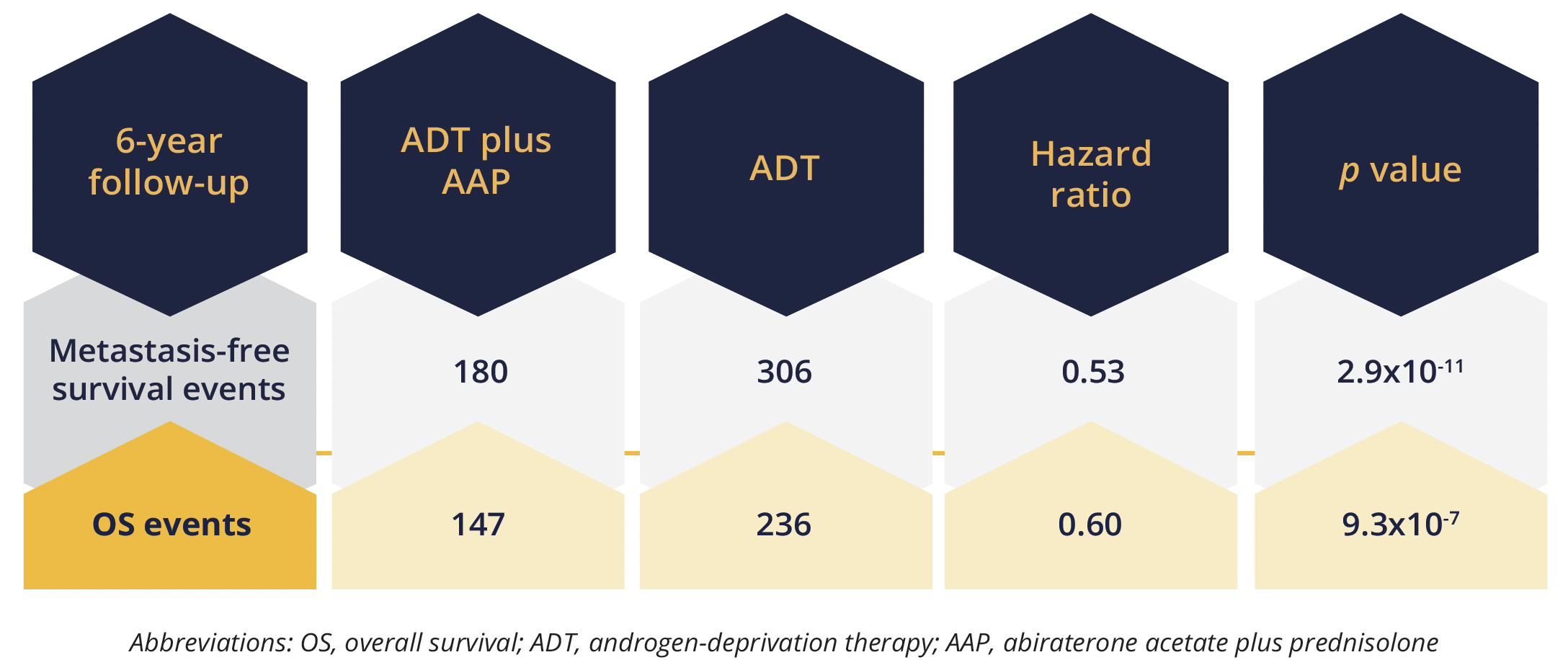
Primary survival endpoints
Presenting author Gerhardt Attard, from University College London in the UK, reported improvements in both metastasis-free survival (no distant metastasis or death from any cause) and overall survival (OS) during 6 years of follow-up with the addition of abiraterone acetate 1000 mg/day plus prednisolone 5 mg, with or without enzalutamide, to standard of care ADT (3 years of ADT plus radiotherapy when indicated) for 2 years. “The magnitude of benefit here was much greater than was previously estimated,” Attard stressed. In all, 986 men were randomly assigned to the abiraterone combination plus ADT and 988 to ADT alone.
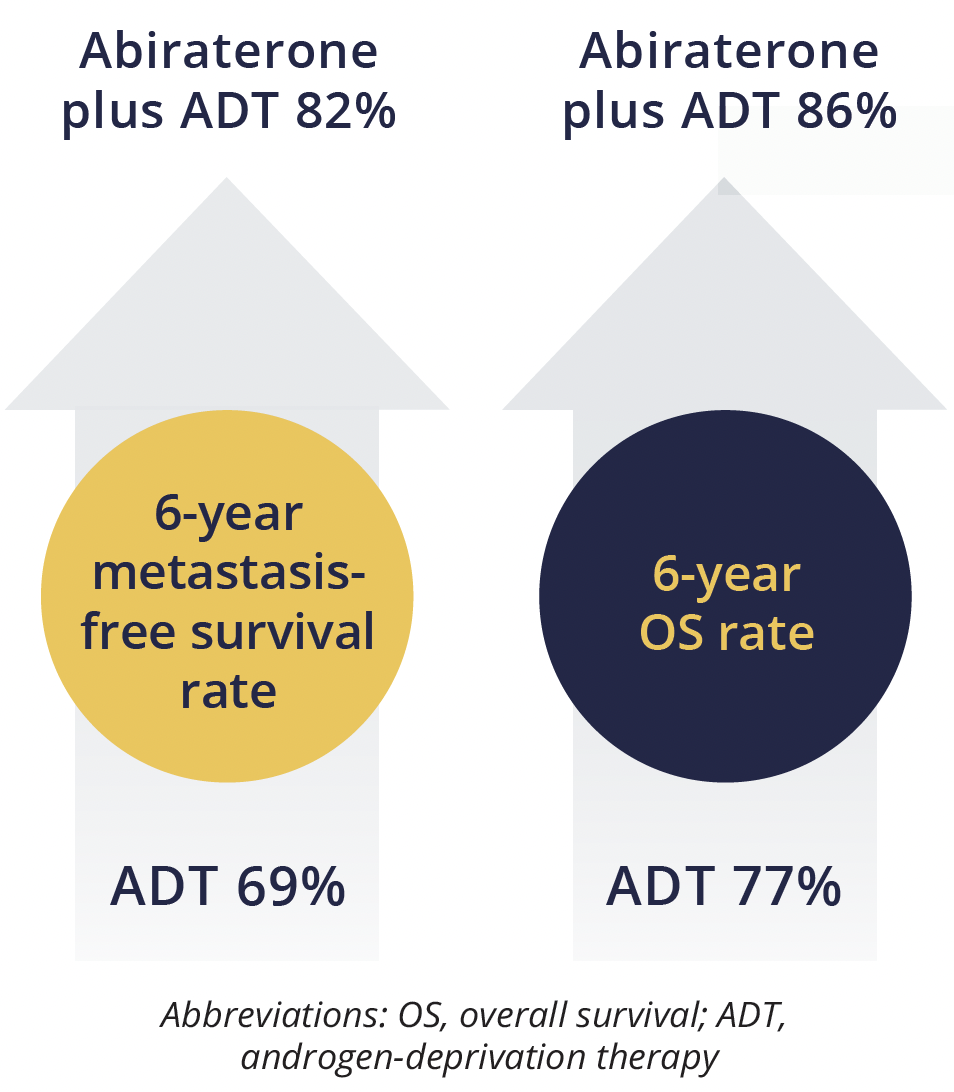
Metastasis-free and overall survival rates
The findings also showed “clear benefit of treatment effect” across all secondary efficacy outcome measures, Attard reported, including 6year prostate cancer specific survival, which improved from 85% to 93% with the addition of abiraterone-based therapy.
He noted that the addition of enzalutamide to abiraterone-based therapy increased toxicity, most commonly grade 3 erectile dysfunction, hypertension, fatigue and grade 3 or 4 transaminitis, but it “had no discernible effect on efficacy”.
There were a total of seven grade 5 events reported in the abiraterone combination group, including one case each of rectal adenocarcinoma, pulmonary haemorrhage and a respiratory disorder, and in those also taking enzalutamide there were two cases each of septic shock and sudden death.
Attard said the findings are “clearly excellent news for patients and for us physicians”, and concluded that abiraterone acetate plus prednisolone should be considered the “new standard of care” in men starting ADT.
Abiraterone, ADT, docetaxel triplet improves survival in de novo metastatic prostate cancer
In more practice-changing findings, the PEACE-1 study (NCT01957436) has shown survival benefits for men with de novo metastatic castration-sensitive prostate cancer with abiraterone given alongside ADT and docetaxel as a triplet.
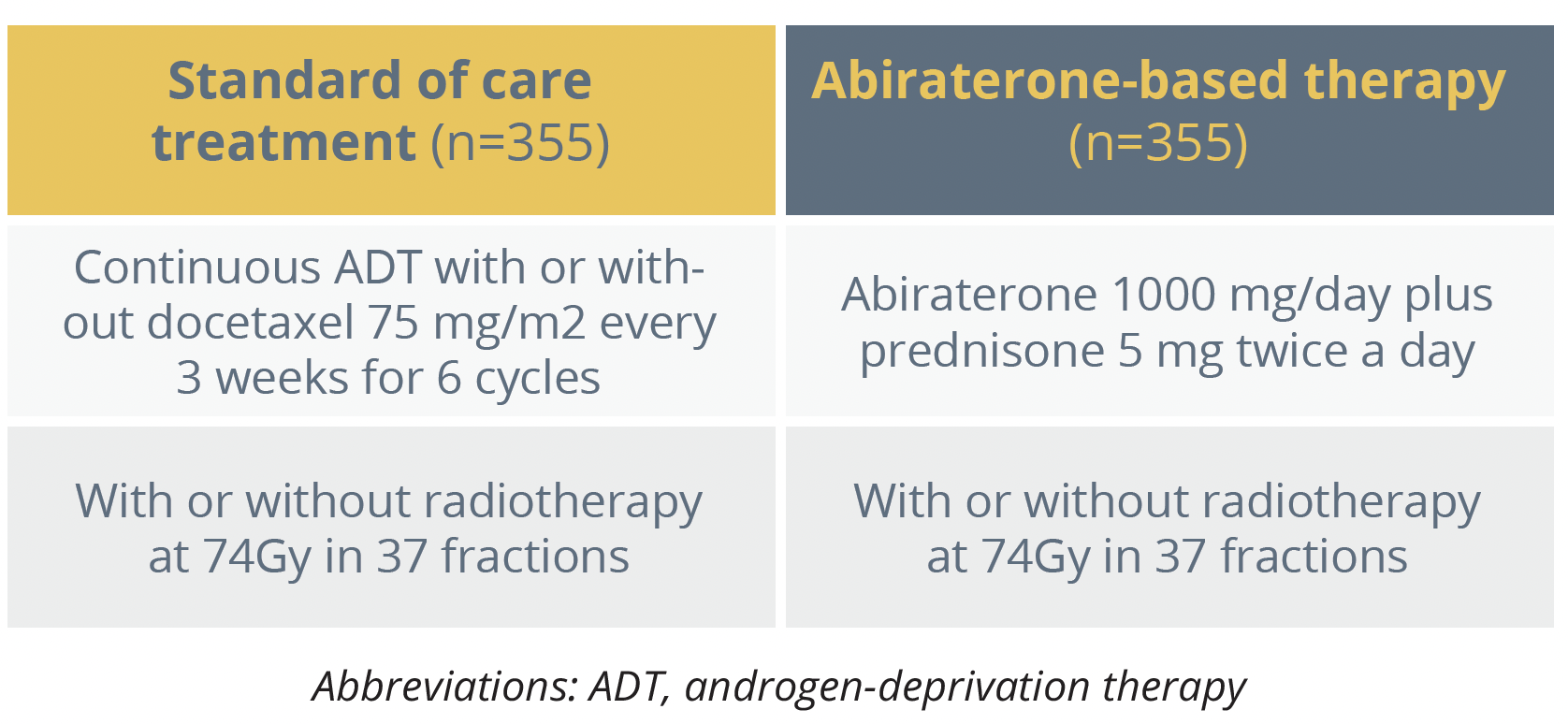
Treatment arms for PEACE-1 study
The presenting author Karim Fizazi, from Institut Gustave Roussy in Villejuif, France, reported a significant 50% improvement in radiographic progression free survival (PFS) with the addition of abiraterone based therapy to ADT compared with standard of care ADT, increasing the median duration from 2.0 to 4.5 years. There was also an OS benefit, with a 25% reduction in the risk of death with the addition of abiraterone treatment versus standard of care alone.
Fizazi noted that for men with a high volume of metastatic burden, which included about 64% of participants, radiographic PFS and OS were reduced by 53% and 28%, respectively, with the abiraterone combination versus standard of care. This translated to a median 2.5year gain in PFS duration and a lifetime gain of 1.6 years. The data for men with low volume disease burden have not yet reached maturity.
The outcome benefits were seen despite 84% of men in the control standard of care group receiving life prolonging treatment following progression, including 81% receiving next-generation hormonal therapy, mostly abiraterone or enzalutamide, Fizazi commented.
“This clearly suggests that early use of these agents is better than deferred use”, he explained.
Among the most common grade 3 to 5 adverse events, neutropenia and febrile neutropenia were evenly balanced between the two treatment arms, whereas “as expected”, hypertension and transaminase increases were seen more often in the abiraterone treatment arm. However, there were no synergistic side effects from the combination, said Fizazi.
“We believe this data is practice-changing: at least men with de novo high volume metastatic prostate cancer should be offered ADT plusdocetaxel plus abiraterone”
Karim Fizazi, Institut Gustave Roussy, Villejuif, France
Discussant Eleni Efstathiou, from Houston Methodist Cancer Center in Texas, USA, welcomed the findings and concluded that while reproducible results are still needed the results of both studies are “truly practice changing”.
TKI treatment breaks proposed for advanced renal cell carcinoma
Research in patients with locally advanced or metastatic renal cell carcinoma presented in the Proffered Paper session has looked at the benefits of a drug-free interval strategy in patients taking tyrosine kinase inhibitors (TKIs) in terms of reduced toxicity and costs.
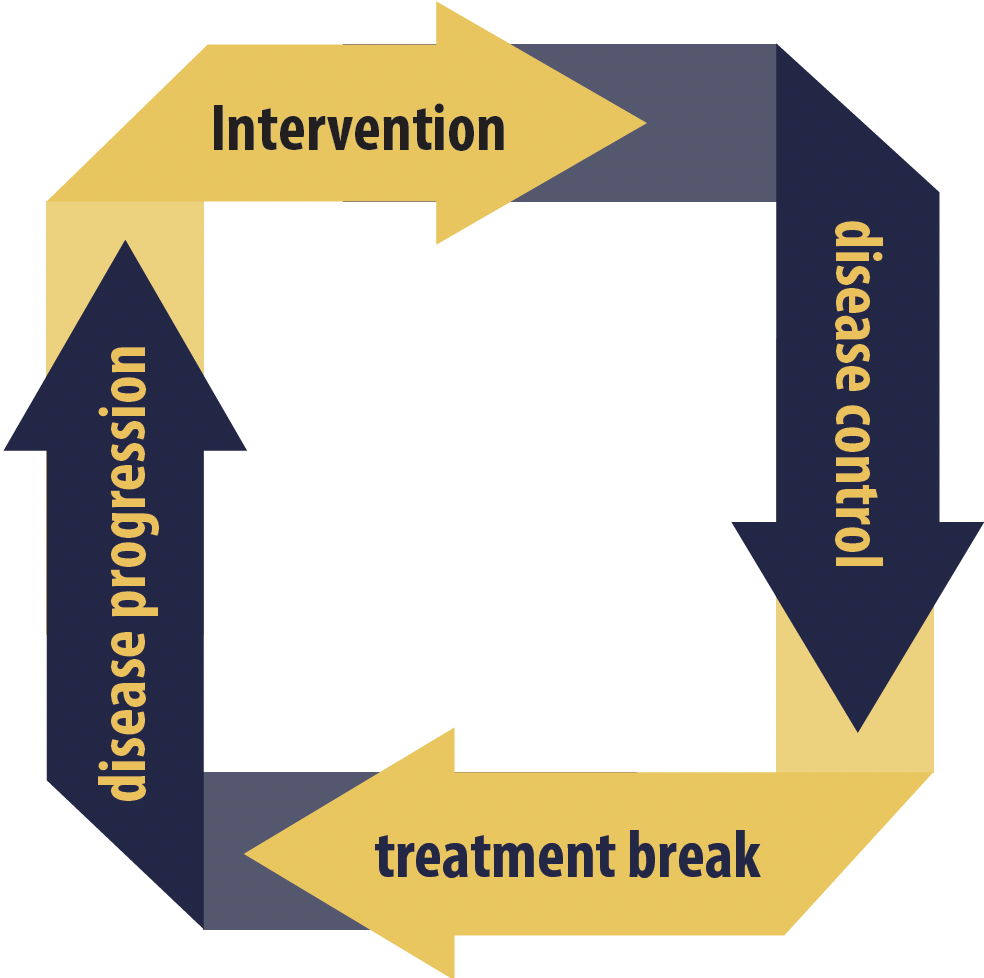
Drug-free interval strategy cycle
Among 919 patients receiving 24 weeks of treatment with 6 week cycles of sunitinib (4 weeks on and 2 weeks off) or daily pazopanib, 458 were randomly assigned to receive intermittent therapy, whereby disease control, determined by quality of life and radiological assessments every 6 and 12 weeks, respectively, prompted a break in treatment. Treatment was restarted if disease progressed again.
The remaining 461 patients made up a control group who received continuous treatment. Presenting author of the STAR study (EudraCT 2011-001098-16) Janet Brown, from the University of Sheffield in the UK, reported that both groups received a similar number of treatment cycles, at a median of 4 to 5. Half of the patients participating in the drug-free interval strategy (DFIS) had the mandatory one break in treatment, with 27.4% taking at least three breaks, up to a maximum of nine, and the median treatment break length was 87 days.
The OS data were inconclusive for noninferiority, which Brown explained was due to reduced power as a result of not enough events being reached. However, the co-primary endpoint of quality-adjusted life years showed a non-inferior effect of the DFIS, equating to no more than a 10% difference.
The strategy was also more cost-effective at 2 years and time to death, disease progression or new systemic treatment, as well as cumulative progression-free interval, showed significant improvements in favour of the DFIS, with hazard ratios of 0.75 and 0.77, respectively.
Brown highlighted that serious adverse events considered to be associated with TKIs occurred less often in patients in the DFIS group and accounted for 39% of the 59 events reported from week 24 onwards, compared with 61% in the continuous treatment group.
“Treatment breaks were acceptable to patients and clinicians, were not detrimental to overall survival or quality of life and had significant cost savings”, she concluded. Discussing the findings, Brian Rini, from Vanderbilt-Ingram Cancer Center in Nashville, Tennessee, USA, agreed that intermittent treatment strategies “should be incorporated into clinical practice”, but that they need to be individualised to the patient. The duration of the treatment break “depends on the inherent growth rate of a given patient’s disease,” he noted.
“At the very least, periodic short breaks off TKI can really help mitigate chronic TKI toxicity”
Brian Rini, Nashville, Tennessee, USA
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.
