
Welcome to ESMO 2021
Delegates seeking the latest in genitourinary cancer research have been treated to a range of key trial findings in the first days of the ESMO Congress 2021, including the first phase 2 results from the NORSE study of erdafitinib plus cetrelimab in advanced urothelial carcinoma, milestone findings for optimal perioperative chemotherapy regimens in muscle-invasive bladder cancer and COSMIC-021 data on cabozantinib plus atezolizumab in metastatic castration-resistant prostate cancer.
Cetrelimab boosts erdafitinib response in patients with advanced urothelial carcinoma
Combining erdafitinib with the PD-1 inhibitor cetrelimab improves objective response rates in cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma and fibroblast growth factor receptor (FGFR) alterations, suggests an interim analysis of the phase 2 NORSE study (NCT03473743).
“The hypothesis for dragging these two drugs together in metastatic urothelial cancer is that erdafitinib may release neoantigens and those neoantigens may be recognized by the immune system prompted by the checkpoint inhibitors […] resulting in what we would hope would be synergy”
Thomas Powles, London, UK
The 19 evaluable patients randomly assigned to receive the pan-FGFR inhibitor erdafitinib 8 mg/day plus intravenous cetrelimab 240 mg every 2 weeks for four cycles and 480 mg every 4 weeks thereafter as first-line therapy achieved an objective response rate of 68%. This compared with an objective response rate of 33% among 18 similar patients receiving first-line treatment with daily erdafitinib alone, Powles reported.
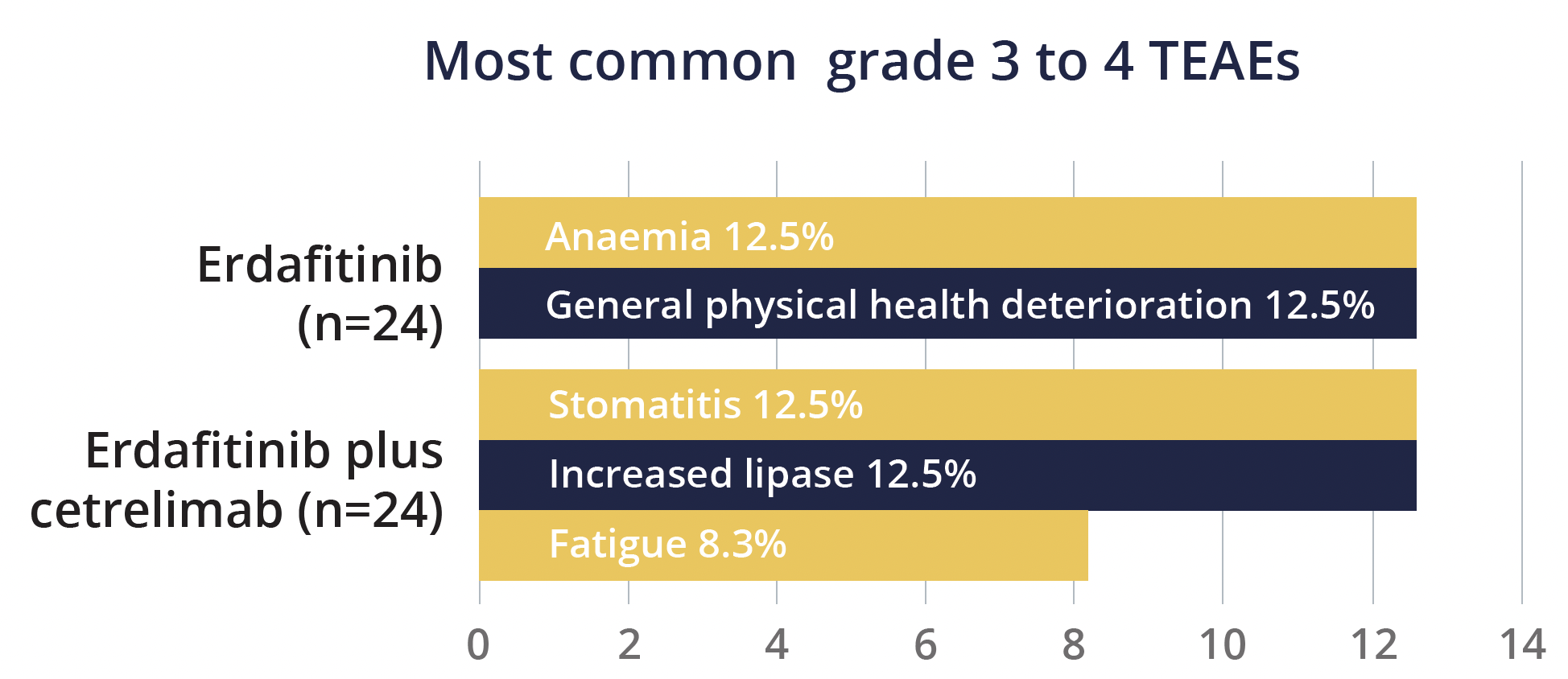
He pointed out that in both sets of patients the vast majority achieved disease control and there were four complete responses among patients in the combination group, compared with one among those receiving monotherapy. Treatment responses were seen in PD-L1-positive and negative patients, although the status was not known for all patients.
The safety profiles were similar for erdafitinib plus cetrelimab and erdafitinib monotherapy, although rates of discontinuation of at least one therapy were greater with combination treatment. Grade 3 to 4 treatment-emergent adverse events (TEAEs) occurred in 50% of patients given erdafitinib plus cetrelimab and 38% of those given erdafitinib.
“The enrolment in NORSE is ongoing and will recruit 90 patients, but when one looks at this data it looks competitive against benchmark historical controls”
Thomas Powles, London, UK
Support for perioperative dose-dense MVAC in muscle-invasive bladder cancer
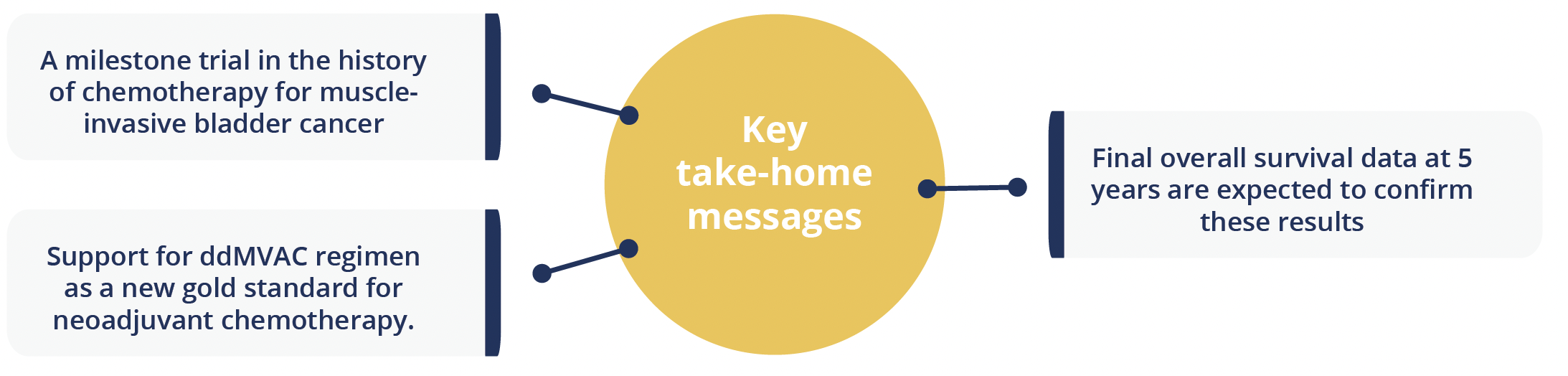
In findings hailed as practice changing, results from the GETUG/AFU V05 VESPER phase 3 trial (NCT 01812369) show improved 3-year progression-free survival (PFS) with dose-dense methotrexate, vinblastine, doxorubicin and cisplatin (ddMVAC) versus gemcitabine and cisplatin (GC) in patients with muscle-invasive bladder cancer.
Among the 248 patients randomly assigned to receive six cycles of ddMVAC every 2 weeks either before or after surgery, the PFS rate at 3 years was 64%, compared with 56% for the 245 patients receiving four cycles of GC every 3 weeks, at a nonsignificant hazard ratio (HR) of 0.77. Presenting author Christian Pfister (Charles Nicolle Rouen University Hospital, France) highlighted that the improvement in PFS with ddMVAC over 40 months of follow-up was significant for the 89% of patients receiving neoadjuvant therapy, at 66% versus 56% with GC, giving an HR of 0.70. The results were inconclusive for the adjuvant group due to the small patient numbers.
The findings also showed a benefit with ddMVAC over GC in time to disease progression, with significant HRs of 0.68 overall and 0.62 for the neoadjuvant treatment group.
Key take-home messages reported by Christian Pfister
Discussant Enrique Grande (MD Anderson Cancer Center Madrid, Spain) pointed out that only 60% of patients completed all six cycles of ddMVAC, compared with 84% of those receiving four cycles of GC, raising a question over whether the number of cycles received needs to be considered.
“VESPER trial supports ddMVAC as the most active regimen in the neoadjuvant approach to muscle-invasive bladder cancer”
Enrique Grande, Madrid, Spain
Cabozantinib plus atezolizumab shows encouraging outcomes in mCRPC
COSMIC-021 (NCT03170960) phase 1b study findings show that combining the tyrosine kinase inhibitor cabozantinib and the PD-L1 inhibitor atezolizumab may offer treatment benefit in men with metastatic castration-resistant prostate cancer (mCRPC).
The results stem from an extended enrolment of men with active mCRPC and radiographic progression in soft tissue despite previous treatment with enzalutamide and/or abiraterone, following encouraging activity in the first 44 patients.
Follow-up of the 132 men over a median of 15.2 months showed that treatment with daily oral cabozantinib 40 mg and intravenous atezolizumab 1200 mg once every 3 weeks resulted in an objective response rate of 23% according to investigator assessment and 15% based on independent radiology assessment. The median time to response was under 3 months in both cases.
The disease control rate was high, at a respective 84% and 81%, which presenting author Neeraj Agarwal (University of Utah, Salt Lake City, USA) said was primarily due to the low numbers of patients who achieved progressive disease as the best overall response. The median duration of response was 6.9 months in both cases, and PD-L1 status, known for 75 patients, was not associated with response.
Agarwal particularly pointed out the antitumour activity in a key subgroup of 101 (77%) men with visceral metastases and/or extrapelvic lymphadenopathy (EPLN), both of which are associated with a poor prognosis.
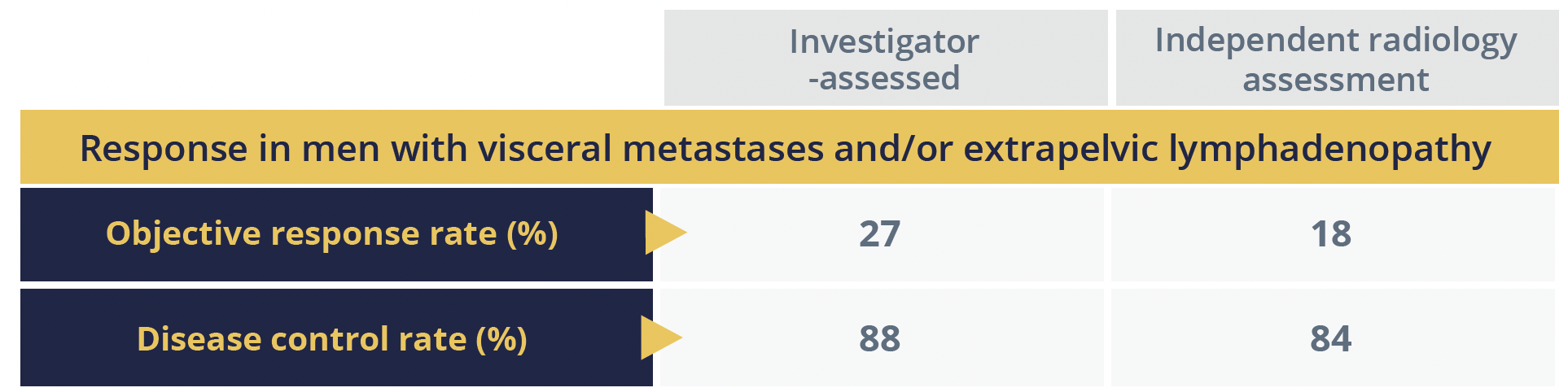
The median duration of PFS was about 5.7 months for the group as a whole and 6.8 months in the visceral metastases/EPLN group, according to independent radiology assessment, while overall survival (OS) was a median of 18.4 months in both groups. Agarwal reported that the safety profile was “manageable, consistent with previously reported data”.
“The combination of cabozantinib and atezolizumab demonstrated encouraging clinical activity in patients with mCRPC, which was confirmed by blinded independent review”
Neeraj Agarwal, Salt Lake City, USA
Prolonged survival with enzalutamide plus ADT in metastatic hormone-sensitive prostate cancer
OS data support previously reported efficacy findings for enzalutamide plus androgen deprivation therapy (ADT) in men with metastatic hormone-sensitive prostate cancer.
The phase 3 ARCHES (NCT02677896) primary analysis demonstrated a significant 61% reduction in the risk of radiographic disease progression with enzalutamide 160 mg/day plus ADT, compared with placebo plus ADT, in 1150 men with metastatic hormone-sensitive prostate cancer.
In the current final OS analysis, conducted over a median follow-up of 44.6 months, there were 356 deaths – 154 in the combination treatment group versus 202 in the ADT group – giving a significant improvement in OS with enzalutamide plus ADT of 34%.
“The early use of enzalutamide with ADT led to a significant delay in the need for subsequent antineoplastic therapy”
Andrew Armstrong, Durham, North Carolina, USA
The survival benefit was maintained irrespective of disease volume, prior local therapy, disease localization, prostate-specific antigen and Gleason score, and it was unrelated to differential receipt of antineoplastic therapies subsequent to the study treatments.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.
