Welcome to ESMO 2021
Treatment programs for advanced colorectal and pancreatic cancers were a focus for the gastrointestinal proffered paper track on the second and third day of this year’s virtual ESMO Congress. The studies assessed whether modifications or additions to well known chemotherapy regimens, such as FOLFOXIRI for advanced colorectal cancer (CRC) or FOLFIRINOX for pancreatic cancer, as well as changes in treatment timing, could impact patient survival.
AtezoTRIBE shows benefit of add-on atezolizumab for metastatic CRC patients
Results from the phase 2 AtezoTRIBE study (NCT03721653), presented by trial investigator Chiara Cremolini (University of Pisa, Italy), showed that atezolizumab can be a useful add-on therapy to FOLFOXIRI plus bevacizumab (bev) for patients with metastatic (m)CRC.
The 218 patients in the study had initially inoperable mCRC and were enrolled to be treated with either eight cycles of FOLFOXIRI/bev (n=73) or FOLFOXIRI/bev plus atezolizumab (n=145) followed by maintenance treatment in a 1:2 ratio. The median follow-up period was 19.9 months.
The primary endpoint of the trial was achieved with a significant improvement in median progression-free survival (PFS) in the add-on atezolizumab group of 13.1 months versus 11.5 months in the FOLFOXIRI/bev group (hazard ratio [HR]=0.69). No major safety issues were observed in the participants.
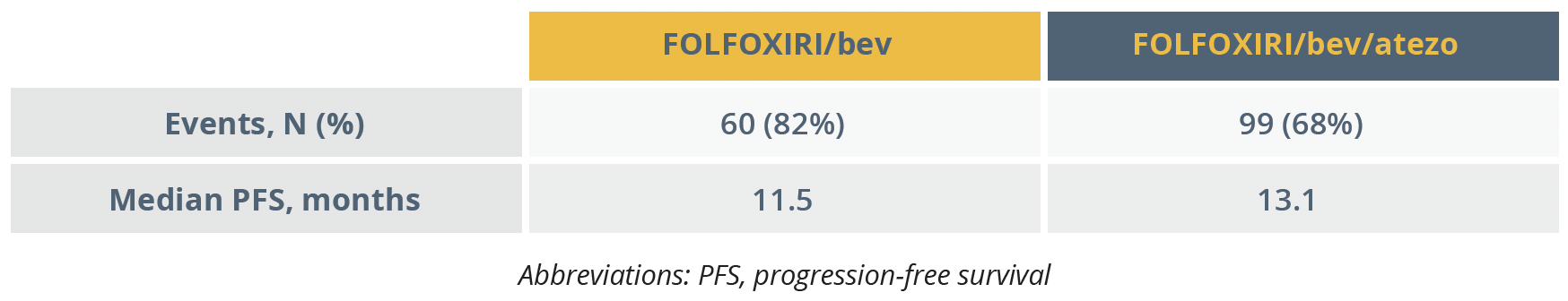
Results for the primary endpoint of progression-free survival
Although the primary endpoint was met, “the magnitude of the progression free survival benefit is clearly heterogeneous according to mismatch repair status,” explained Cremolini.
Individuals with deficient mismatch repair (dMMR) had a better response to the atezolizumab add on therapy than those with normal mismatch repair (pMMR), with only 25% of the dMMR group versus 71% of the pMMR group treated with FOLFOXIRI/bev/atezo experiencing progression during follow-up (HR=0.11). However, there was still a trend towards benefit seen with add-on atezolizumab in the pMMR group (HR=0.78).
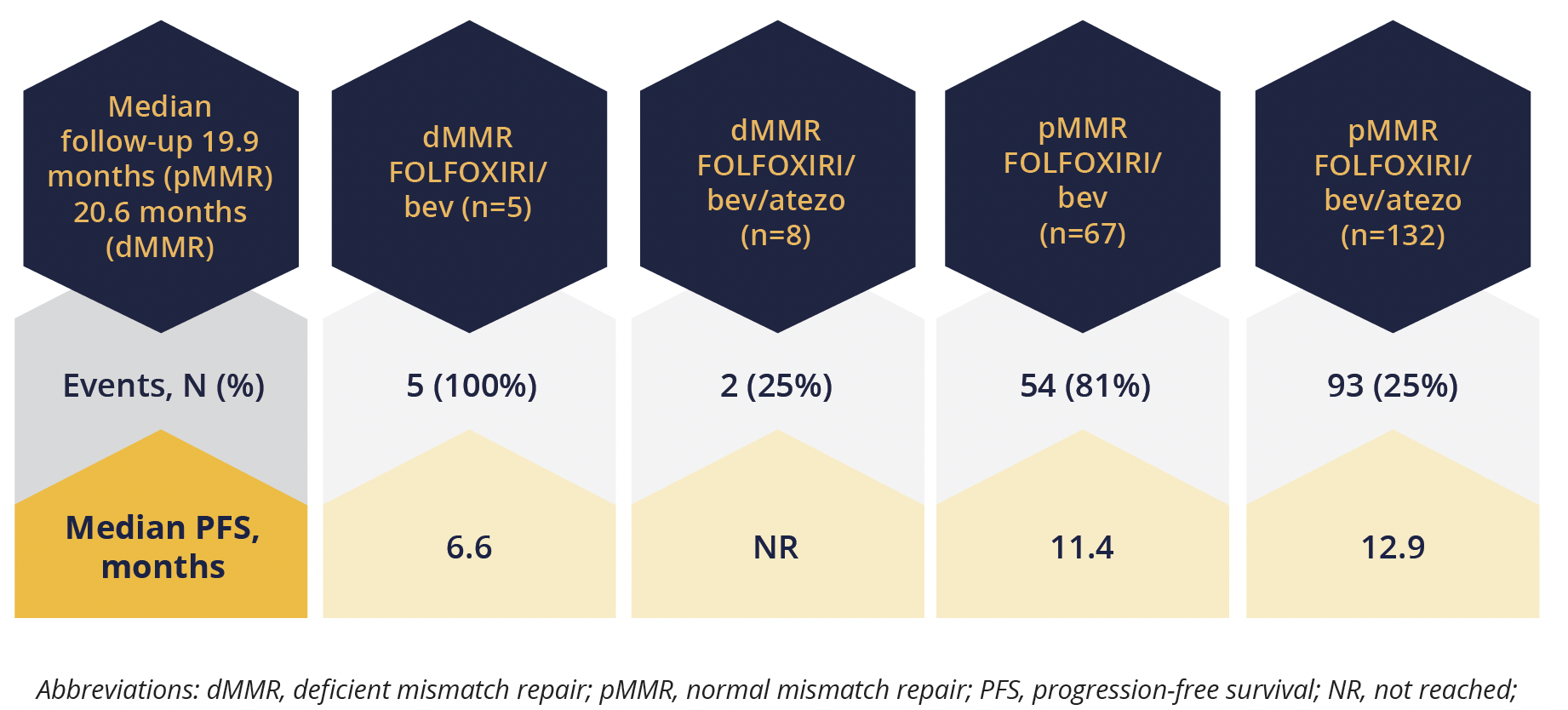
Progression-free response rates according to mismatch repair status
“Biomarkers are currently under investigation and there is a program of translational analysis ongoing to identify potential predictors of benefit from the addition of atezolizumab,”
Chiara Cremolini, Pisa, Italy
The discussant for this study, Dominik Modest (Charité Universitätsmedizin Berlin, Germany), acknowledged the achievement of the primary endpoint, but pointed out that the overall response rate was no different between the FOLFOXIRI/bev and FOLFOXIRI/bev/atezo groups, at 64% versus 59%, respectively. He said he is sceptical that the results will change clinical practice.
Chemotherapy before surgery should be considered for pancreatic cancer patients
Even operable pancreatic cancer has a poor outcome after surgery. The NEONAX trial (NCT02047513) assessed whether a regimen of gemcitabine (1000 mg/m2) and nab-paclitaxel (125 mg/m2) given either before and after surgery, or after surgery could help improve disease-free survival (DFS) in the trial participants.
The trial was carried out at 22 different sites in Germany. The 63 patients assigned to Arm A had six cycles of treatment given as two in the 4 weeks prior to surgery to remove their tumour and four in the 12 weeks following surgery. The 64 assigned to Arm B had all six cycles of treatment in the 12 weeks following surgery.
The primary endpoint of an improvement in DFS from 38% to 55% in both arms at 18 months was not met. In the modified intention-to-treat (mITT) population DFS at 18 months was 32.2% in Arm A and 41.4% in Arm B.
However, there was still a trend towards benefit seen with add-on atezolizumab in the pMMR group (HR=0.78).
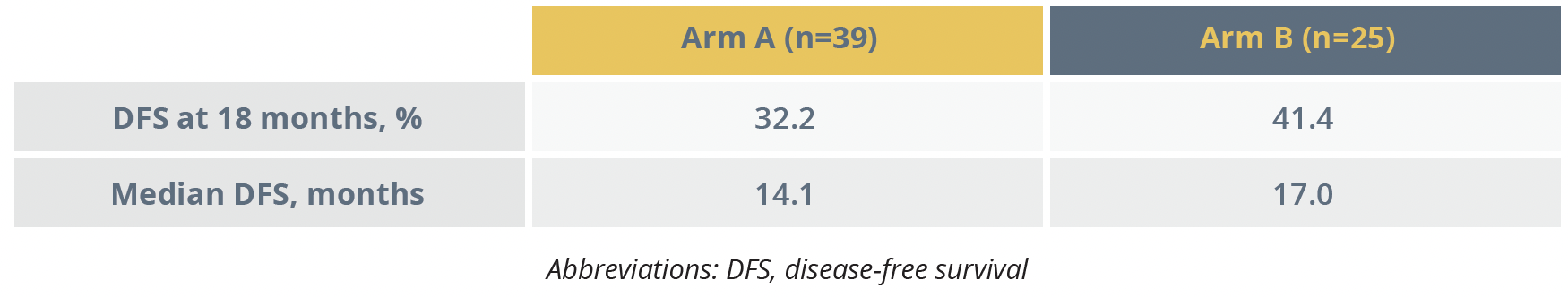
Disease-free survival outcomes in modified intention-to-treat group
Although the primary endpoint was not achieved, the median DFS in the full ITT population was better in Arm A than Arm B, at 11.4 versus 5.9 months, respectively. Arm A also had a good overall tumour response rate of 28.9% and a low tumour progression rate of 6.7%.
“A larger group of patients can benefit when chemotherapy is given prior to surgery,”
Thomas Seufferlein, University of Ulm, Germany
“Therefore, we conclude that neoadjuvant chemotherapy [before surgery] should be considered as a standard for novel clinical trials particularly with respect to examining the duration and intensity of preoperative chemotherapy, as it is done in various novel trials that are ongoing.”
Updated phase 3 results confirm modified FOLFORINOX benefits after pancreatic cancer surgery
Updated 5 year results from the Unicancer PRODIGE 24/CCTG PA6 trial (NCT01526135), which reported 3 year results in 2018, confirm that modified FOLFORINOX chemotherapy after pancreatic tumour removal surgery results in significantly improved DFS compared with gemcitabine treatment.
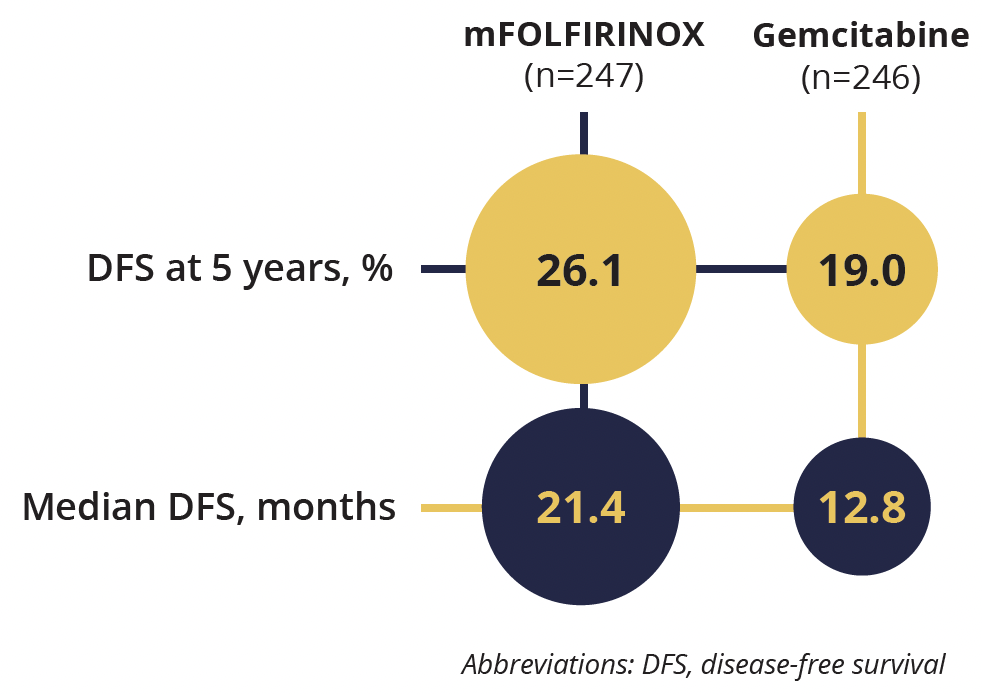
Disease-free survival rate and duration
Overall, 493 patients were followed up for a median of 69.7 months. After surgery to remove pancreatic ductal adenocarcinomas, the patients received either modified FOLFORINOX, without bolus fluorouracil to decrease the incidence and severity of hematologic toxic effects and diarrhoea, every 14 days for 12 cycles (n=247), or 1000 mg/m2 of gemcitabine on days 1, 8 and 15 for six cycles (n=246).
The 5 year DFS rate for the group taking modified FOLFORINOX was 26.1% versus 19.0% with gemcitabine (HR=0.66) meeting the primary endpoint of the study. Various secondary endpoints were also met including overall and metastasis-free survival.
This additional follow-up study was completed because when the independent data and safety monitoring committee for the trial recommended early analysis and publication of results in February 2018, only 314 of the 342 events required for final analysis had occurred.
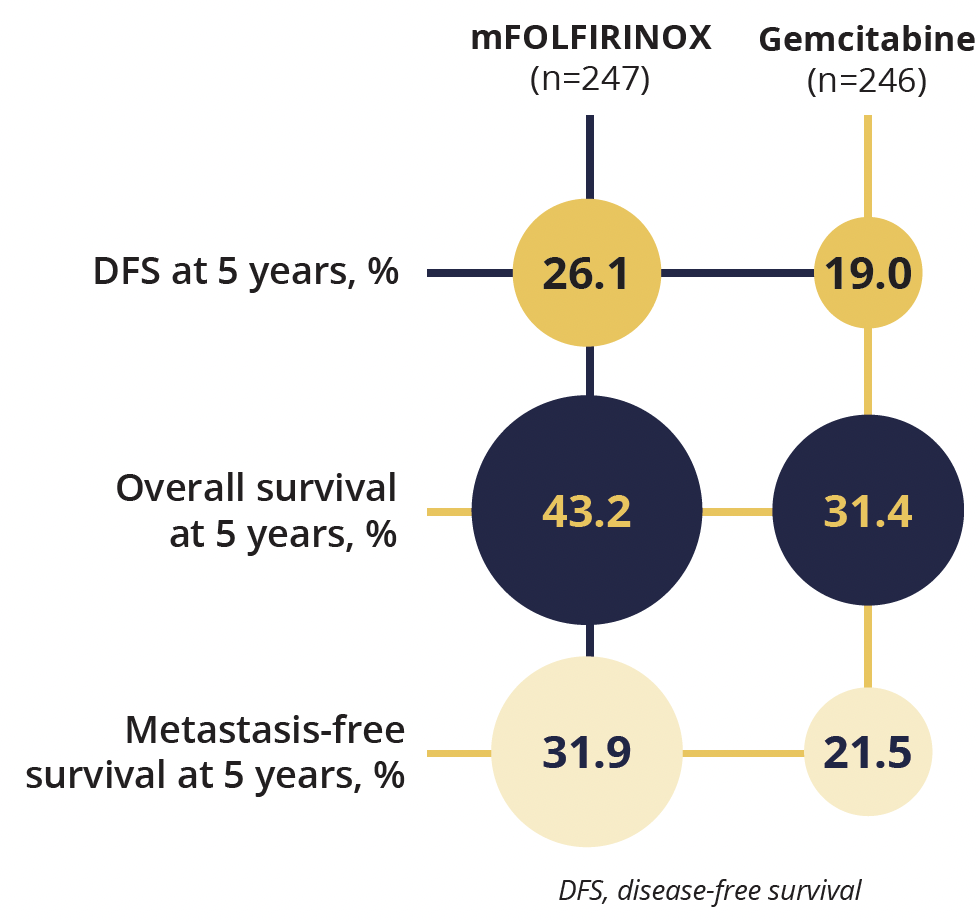
Metastasis-free survival and overall survival outcomes
Notably, it was important for patients to complete all their treatment cycles in this study. Across both groups, 5-year survival was 41.9% if all chemotherapy was received and only 27.4% if treatment was incomplete. “For all outcomes including overall survival we identify the completion of all cycles as an important prognostic factor,” said presenting investigator Thierry Conroy (Institut de Cancérologie de Lorraine, France).
“These major data confirm that modified FOLFORINOX is the most efficient regiment in the adjuvant [post surgical] setting for fit patients.”
Thierry Conroy, Institut de Cancérologie de Lorraine, France
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.
