Welcome to ESMO 2021
The second half of the ESMO Congress 2021 included some interesting trial results for gastrointestinal cancer therapies. These included a look at the efficacy of the KRAS-targeting drug adagrasib in colorectal cancer patients, the use of nivolumab as a first-line treatment for several types of advanced gastric cancer, and a look at new approaches to treating rectal cancer.
Cautious optimism for patients with KRAS-mutated colorectal cancer
Jared Weiss (University of North Carolina-Chapel Hill, USA) presented results from the KRYSTAL-1 phase 1/2 study (NCT03785249) trialing the experimental KRAS-G12C inhibitor adagrasib, which is being tested to treat both lung cancer and advanced colorectal cancer (CRC) with the G12C mutation in the KRAS gene. This is currently a group with limited treatment options, as CRC with this kind of mutation often does not respond to other commonly used therapies such as the antibody therapy cetuximab. In this study, 46 patients received adagrasib (600 mg twice daily) alone and 32 adagrasib plus cetuximab and were followed up for a median of 7–9 months.
“KRAS-G12C mutations occur in approximately 3–4% of colorectal cancer, act as oncogenic drivers and are strong negative predictors of cetuximab efficacy,”
Jared Weiss, USA
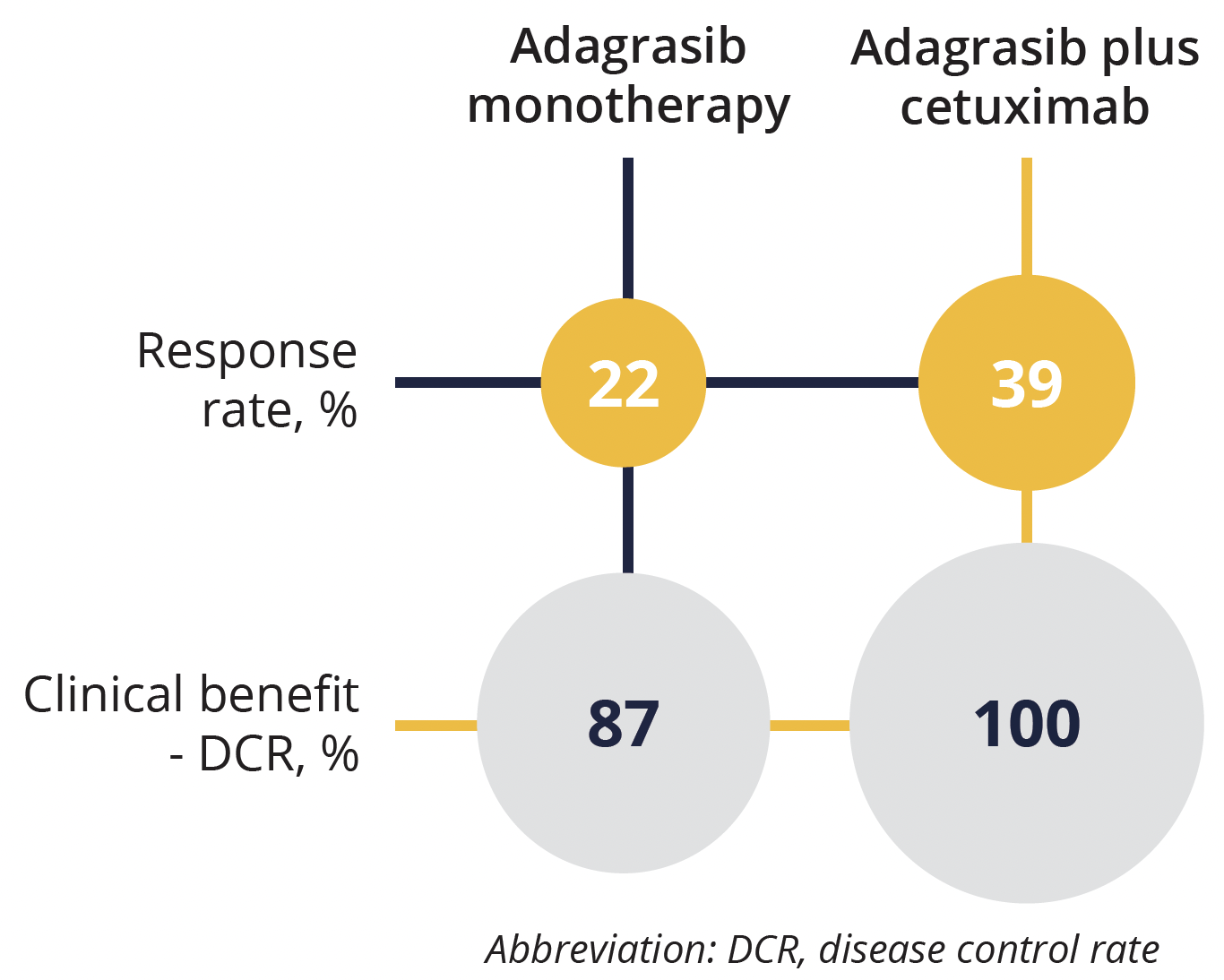
Response results for adagrasib plus cetuximab versus adagrasib monotherapy
At the time of the analysis around 71% of the patients were still being treated, so the data are somewhat immature. Overall, 22% of patients responded to adagrasib alone and 39% to adagrasib and cetuximab. Broad disease control was seen in 87% of the adagrasib group and 100% of the combined treatment group. Median progression-free survival in the adagrasib group was 5.6 months. The overall response was better in the combination therapy group, but more side effects (skin related) were observed. However, while frequent, the side effects were not life threatening in either group.
As noted by the discussant of this session Federica di Nicolantonio (University of Turin, Italy) adagrasib seems to be more effective at treating non-small cell lung cancer than advanced CRC with this mutation, with a recent reported overall response rate of 45% for monotherapy.
“The response rate to another KRAS inhibitor sotorasib, is actually higher in non-small cell lung cancer compared to colorectal cancers,” said di Nicolantonio. “And based on the previous data that was presented last year and what you heard today; you actually see that the same seems to be true also for adagrasib.”
Long awaited new first-line treatment option for gastric cancer
The phase 3 CheckMate 649 study (NCT02872116) trialed nivolumab plus chemotherapy or ipilimumab for first-line treatment of advanced gastric cancer, gastroesophageal junction cancer or oesophageal adenocarcinoma in HER2-negative patients.
The study randomly assigned 2031 patients to receive either nivolumab plus chemotherapy (XELOX or FOLFOX), nivolumab plus ipilimumab or chemotherapy alone. Nivolumab plus chemotherapy led to significantly improved overall survival at 12 months, as published in The Lancet earlier this year, and was approved for this indication by the US FDA earlier this summer.
“It already changed practice for patients with metastatic gastric, oesophageal and gastroesophageal -junction adenocarcinoma,”
Yelena Janjigian, New York, USA.
Improvements in overall and progression-free survival with nivolumab plus chemotherapy versus chemotherapy alone seen at 12 months were maintained at 24 months, at 14.4 months versus 11.1 months and 8.1 versus 6.1 months, respectively.
However, the same was not true for the ipilimumab arm, which failed to improve overall survival (11.2 vs 11.6 months for combination therapy vs chemotherapy) and actually reduced progression-free survival (2.8 months) compared with chemotherapy alone (6.3 months).
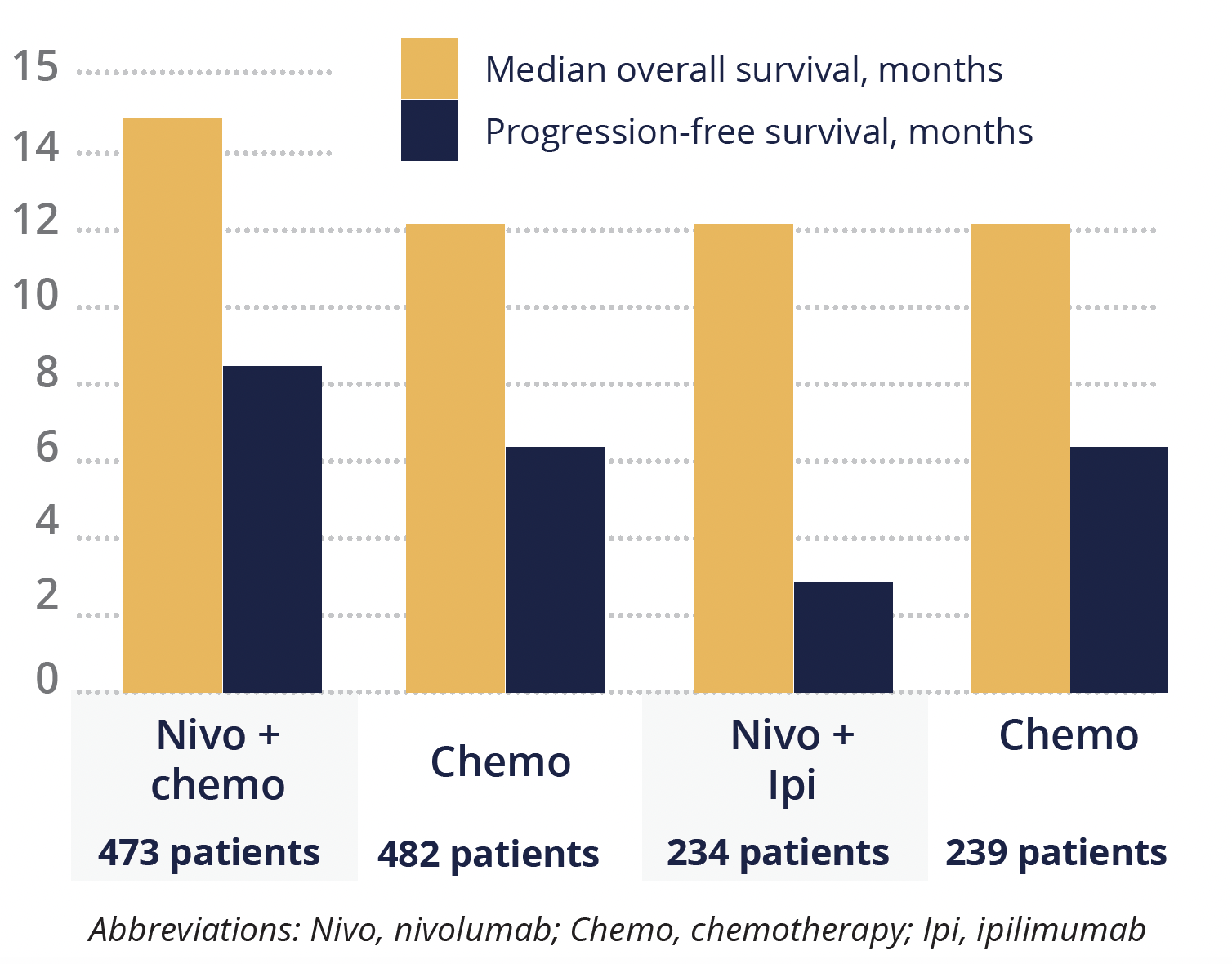
Overall and progression-free survival outcomes
“We have been waiting a long time for this success. The data of the ToGA study for HER2-positive patients, was 10 years ago and since then, in the first line setting of gastric cancer, we have seen many studies but all with negative outcomes,” emphasised discussant Florian Lordick (University of Leipzig, Germany).
“Checkmate 649 is the first randomized global phase 3 study for patients with HER2-negative metastatic gastric cancer that is a positive study,” he added, although he acknowledged that the “elephant in the room” of the failed ipilimumab arm needs to be analysed further.
Support for avoiding radiotherapy in patients with advanced rectal cancer
The phase 3 CONVERT study (NCT02288195) was presented by Pei-Rong Ding (Sun Yat-sen University Cancer Center, Guangzhou, China) and assessed whether chemotherapy could offer similar or better results than chemotherapy and radiotherapy combined for patients with locally advanced rectal cancer with uninvolved mesorectal fascia.
“Neoadjuvant chemoradiotherapy is currently the standard practice for locally advanced rectal cancer. However, the survival benefit has not been confirmed,” explained Ding. “Furthermore, neoadjuvant chemoradiotherapy is associated with radiation toxicity, increased surgical difficulties and morbidity and impaired quality of life.”
Overall, 663 patients were recruited to take part in the study; 331 were randomly assigned to receive four cycles of oxaliplatin and capecitabine alone (nCT) and 332 received chemoradiotherapy with capecitabine (nCRT).
The pathological complete response rate was 11.0% in the nCT arm vs 13.8% in the nCRT arm. Most of the patients went on to require surgery, but two nCT patients had a complete response and did not need surgery compared with five in the nCRT arm.
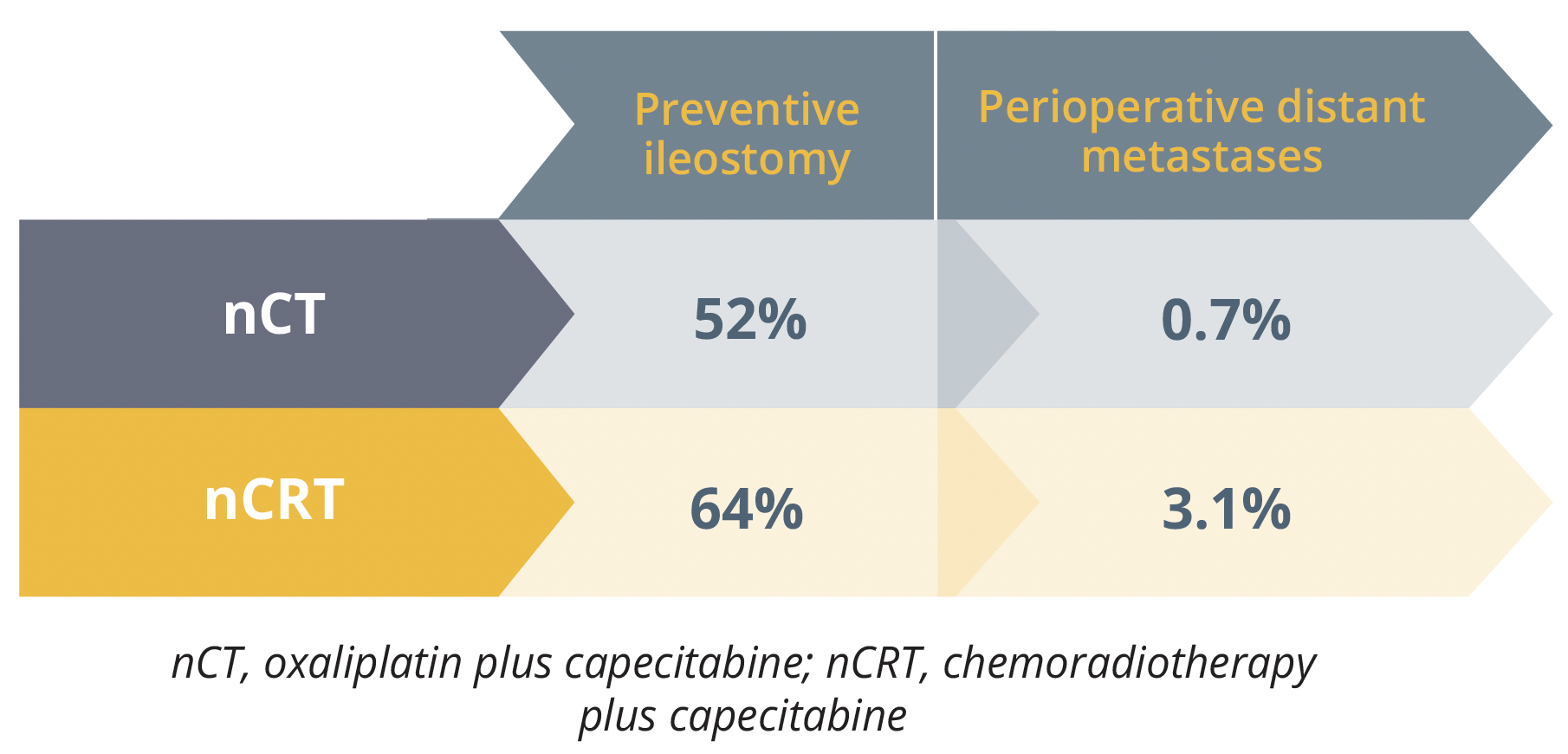
Surgical outcomes for the nCT and nCRT arms
Perioperative distant metastases were seen less in the nCT compared with the nCRT arm, at 0.7% versus 3.1%, respectively. Similarly, fewer preventive ileostomies were needed in the nCT arm compared with the nCRT arm, at 52% versus 64%. Ding concluded that the two treatment regimens achieved similar results, with some improvements seen in the nCT group in terms of preventive ileostomy and metastases, and suggested that nCT might be a good alternative to nCRT if these results are validated by additional long-term studies.
Discussant Julien Taieb (University of Paris, France) agreed that reducing radiotherapy is a good goal for patient wellbeing, but said he would like to see more follow-up data including quality of life and side effects data for this study. He also encouraged trialling this in a larger and more diverse population of patients.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.


