
Welcome to ESMO 2021
The third and fourth days of the ESMO Congress 2021 saw exciting research presented in the haematology space, including the use of gene signatures to predict transformation risk, anti-tumour activity of the anti-PD1 antibody geptanolimab in relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), and the use of novel PI3K inhibitors, either in combination or as monotherapy, for the treatment of lymphoma.
Gene signature predicts risk of transformation in patients with follicular lymphoma
A prognostic signature, which allows patients with follicular lymphoma (FL) to be stratified according to their risk of transformation, has now been validated, with genomic analysis confirming the association of mutated genes with a higher risk of transformation.
Comparative analysis of diagnostic samples from 21 pre-transformed (pre-tFL) and 30 non-transformed (ntFL) FL patients revealed that pre-tFL showed more mutations than ntFL samples. In addition, more genetic complexity at diagnosis was associated with transformation, with a lower variant allele frequency (VAF) in pre-tFL samples than in ntFL (24 vs 30%), resulting in a higher proportion of subclonal mutations (<20% VAF) per sample (38 vs 24%).
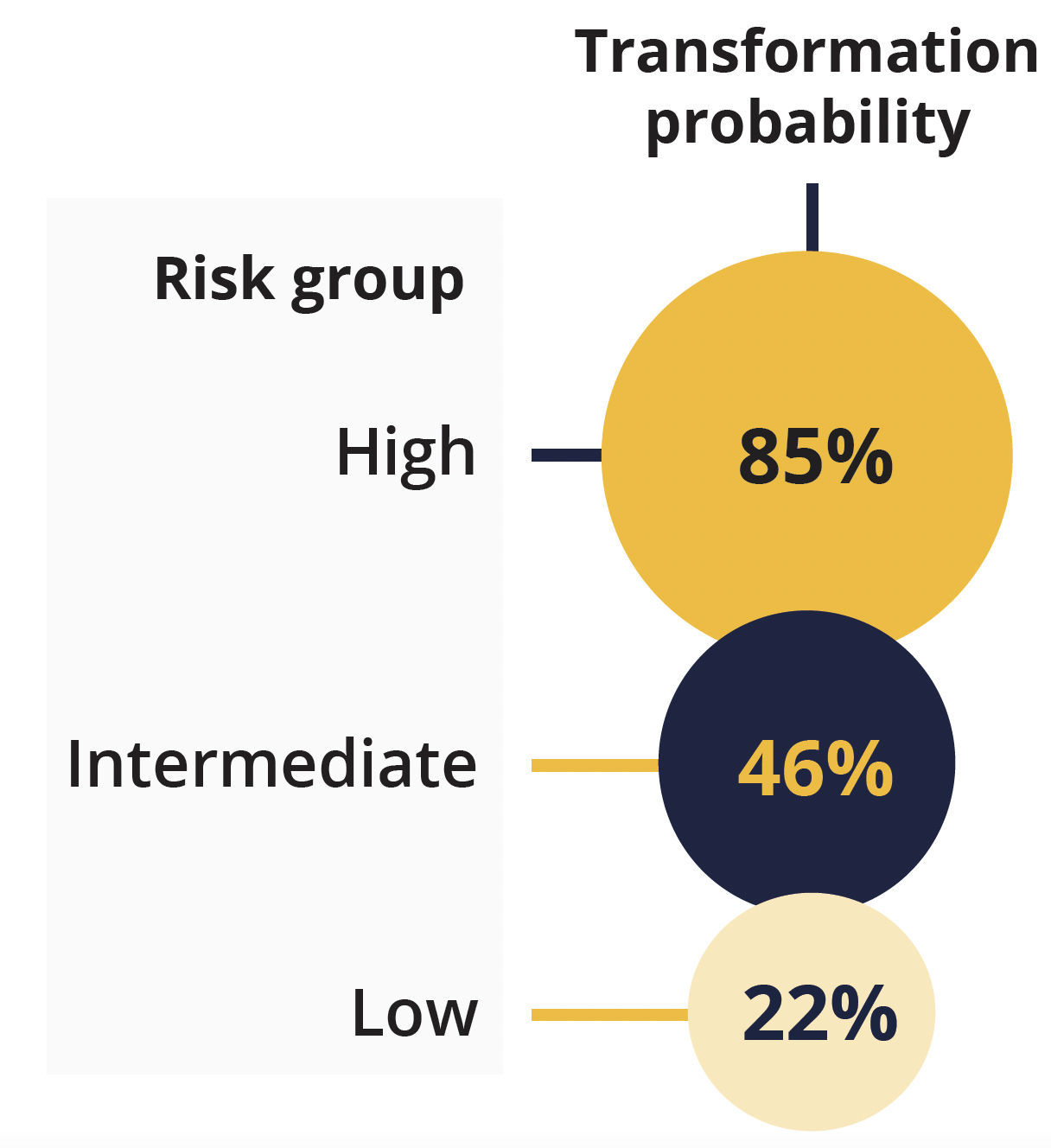
Transformation probability according to risk group
Presenting author Ismael Fernandez-Miranda (Instituto de Investigación Sanitaria Puerta de Hierro-Segovia de Arana, Majadahonda, Spain) explained that mutations in HIST1H1E, NOTCH2, IRF8 and UBE2A were associated with transformation. Combining these with the Follicular Lymphoma International Prognostic Index enabled classification of the samples into three risk groups, with distinct transformation probabilities at 5 years.
Fernandez-Miranda concluded that a predictive model with both mutational status and clinical risk factors can improve risk stratification and could be useful for identifying which patients with FL are at an increased risk of transformation.
Encouraging anti-tumour activity with geptanolimab in relapsed/refractory PMBCL
Preliminary data from a phase 2 GB223-003 trial (NCT03639181) show that the novel anti-PD1 antibody geptanolimab offers good anti-tumour activity in Chinese patients with relapsed/refractory PMBCL, with a manageable safety profile.
In the current analysis of 25 patients, the overall response rate (ORR) was 64%, including seven patients (28%) with a complete response (CR) and nine patients (36%) with a partial response.
Presenting author Yuankai Shi (Chinese Academy of Medical Sciences – National Cancer Center, Beijing) also highlighted that geptanolimab offers a sustained and meaningful survival benefit to patients, with median progression-free survival (PFS) and overall survival (OS) having yet to be reached over median follow-up periods of 10.97 and 20.50 months, respectively.
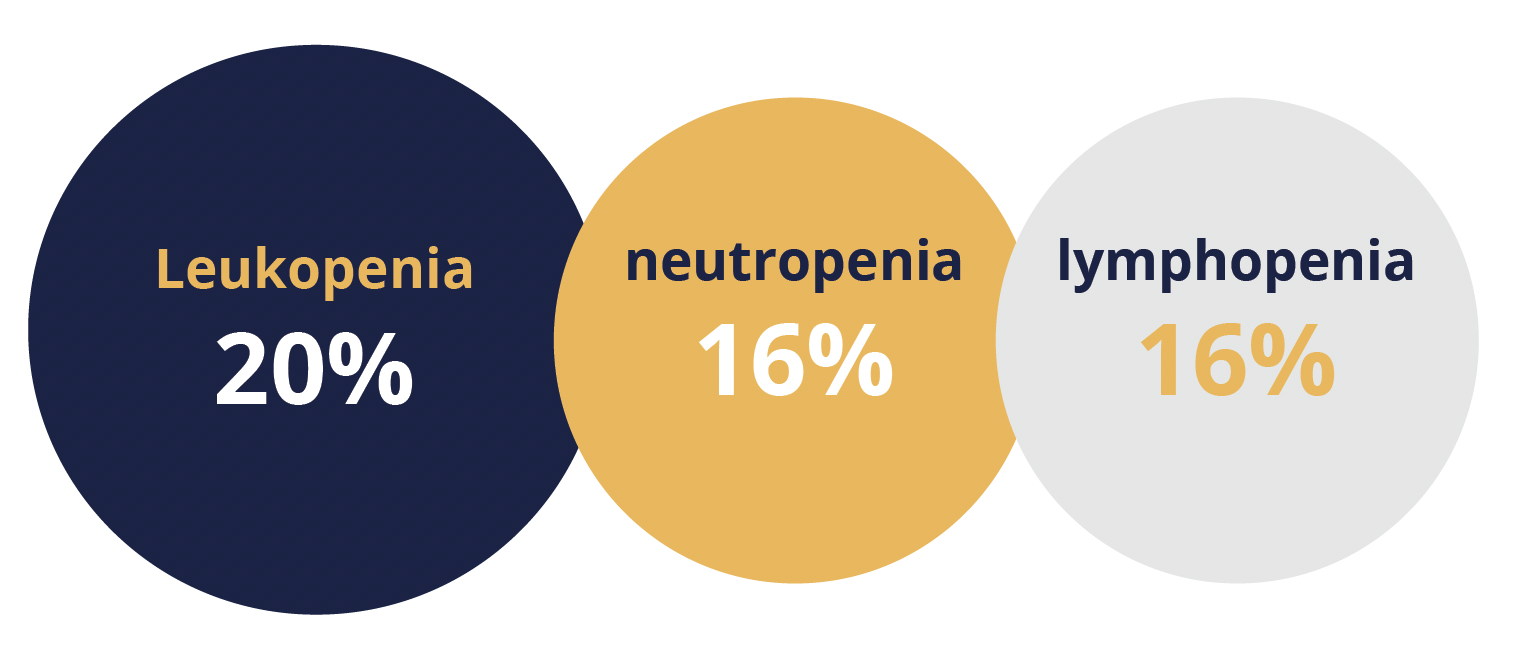
Most common treatment-related adverse events
He noted that “the most common treatment-related [TRAEs] were similar to other immune checkpoint inhibitors”. Specifically, the most common TRAEs of grade 3–4 were leukopenia (20%), neutropenia (16%) and lymphopenia (16%). Geptanolimab may potentially be a new treatment option for Chinese patients with relapsed or refractory PMBCL, concluded Shi.
Combining copanlisib with rituximab shows promise for relapsed MZL
A subset analysis of data from the phase 3 CHRONOS-3 study (NCT02367040) has shown that the PI3K inhibitor copanlisib used in combination with rituximab offers superior efficacy versus placebo plus rituximab in patients with relapsed marginal zone lymphoma (MZL), including nodal MZL.
The study investigators previously reported a 48% risk reduction in disease progression or death with copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma.
The current analysis, conducted at a median follow-up of 18.0 months, showed that the combination treatment significantly improved PFS compared with rituximab monotherapy in all patients with MZL (22.1 vs 11.5 months), equivalent to a 52% reduction in risk of disease progression or death. Presenting author Muhit Özcan (Ankara University School of Medicine, Turkey) highlighted that while statistical conclusions were limited by the small number of patients in each MZL subtype group, particularly in the placebo plus rituximab arm, numerical improvements in PFS were observed across all three subtypes, with significant improvements seen in patients with nodal and extranodal MZL.
Copanlisib plus rituximab also significantly improved ORR overall and in the nodal and extranodal subgroups, but not in the splenic subgroup, compared with monotherapy. Median OS is not yet evaluable.
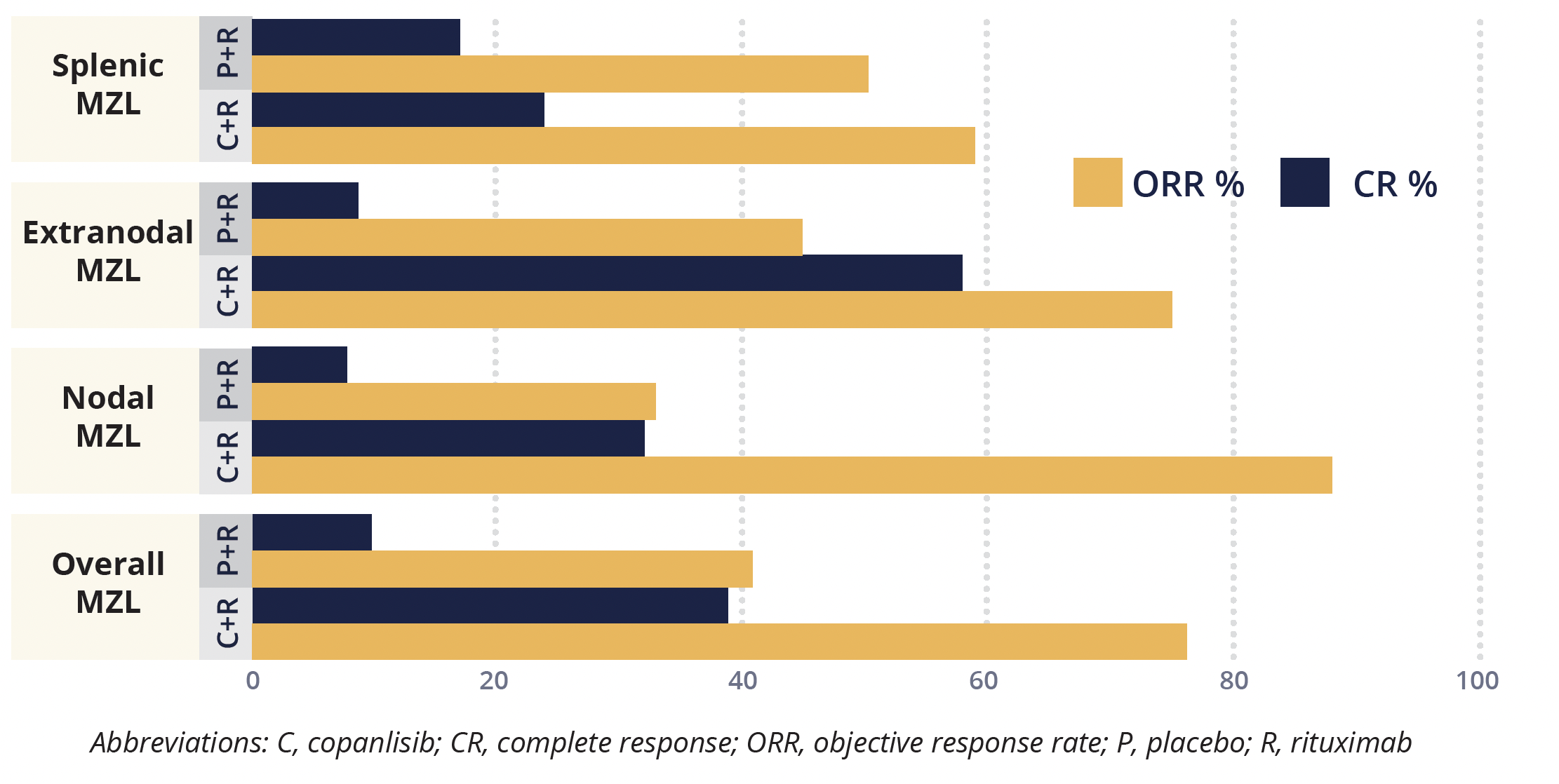
Objective response and complete response rates in subgroups of patients with marginal zone lymphoma
The safety profile of copanlisib plus rituximab was generally consistent with the known safety profile of each treatment as monotherapy. The most common grade 3–4 treatment-emergent adverse events (TEAEs) with copanlisib plus rituximab were hyperglycaemia (53.9%), hypertension (44.6%), and neutropenia (18.5%), while with placebo plus rituximab these were hyperglycaemia (10.3%), hypertension (10.3%) and neutropenia (13.8%). Serious TEAEs of all grades were more common for patients receiving copanlisib plus rituximab (56.9%) than placebo plus rituximab (27.6%).
Özcan concluded that copanlisib is the first PI3K inhibitor to be safely combined with rituximab in a phase 3 setting in patients with relapsed MZL, representing a new therapeutic strategy.
Novel class I PI3Kδ inhibitor shows promising clinical activity in relapsed/refractory BCL
Preliminary data from a phase 1 study (NCT03128164) show HMPL-689, a highly selective PI3Kδ inhibitor, to have promising single-agent activity in Chinese patients with relapsed/refractory B-cell lymphoma (BCL), with high ORR and CR rates noted, particularly for patients with FL.
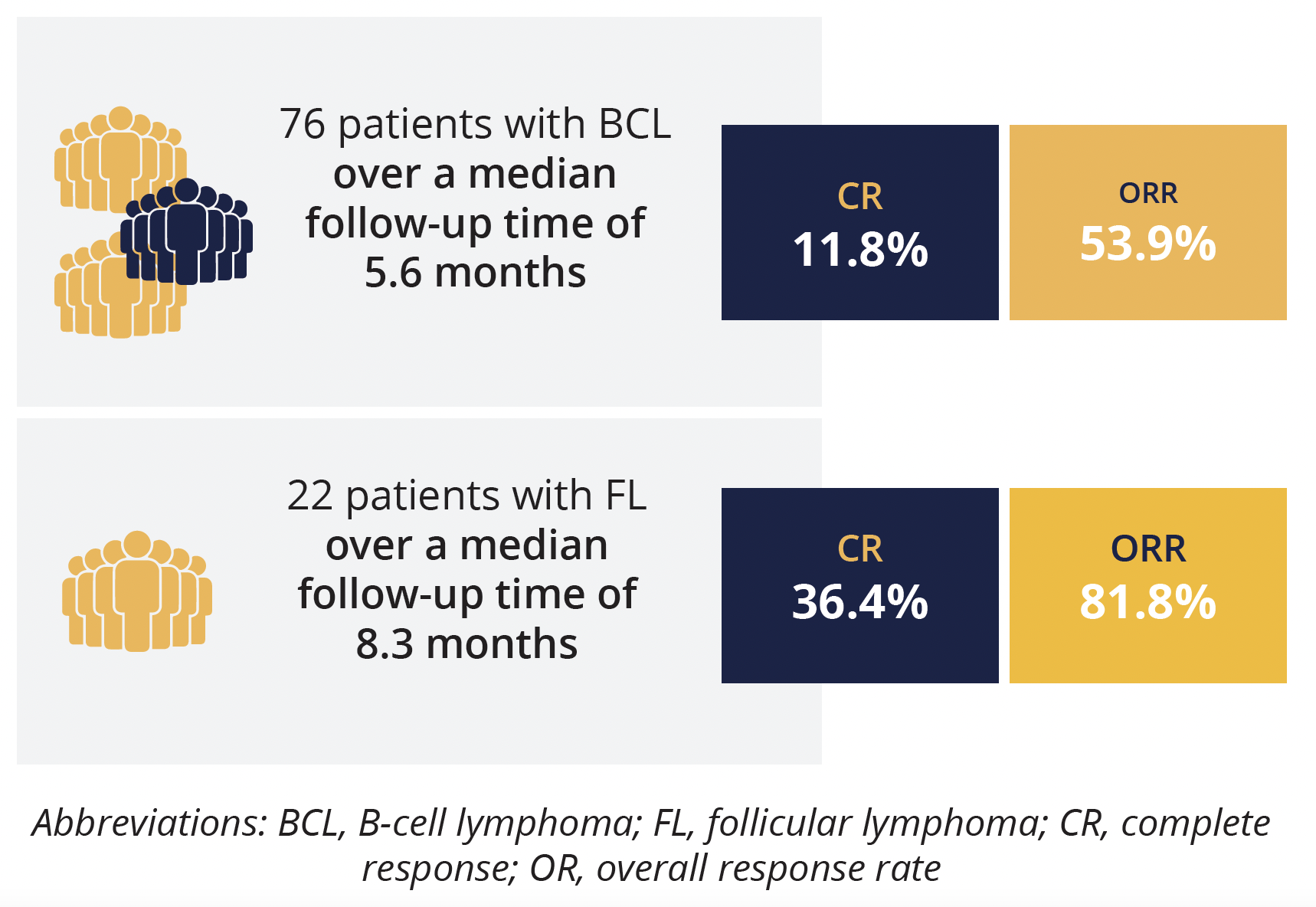
Response rates in B-cell lymphoma patients and subgroup with follicular lymphoma
The study investigators previously showed encouraging preliminary efficacy in patients with relapsed/refractory lymphoma in the dose-escalation stage of the study, with the present data taken from the use of HMPL-689 at the recommended phase 2 dose of 30 mg four times daily. In the efficacy evaluable population (76 patients), with a median follow-up time of 5.6 months, ORR was 53.9% and CR was 11.8%. However, presenting author Junning Cao (Fudan University Cancer Center, Shanghai, China) highlighted that the treatment response was better in the 22 patients with FL – over a median follow-up time of 8.3 months, ORR was 81.8% and CR rate was 36.4%.
HMPL-689 was well tolerated, with a manageable safety profile – the most frequent TEAE was decreased neutrophil count (28.9%) and the most frequent grade 3 TEAE was pneumonia (13.3%). Of note, there were few episodes of grade 3 diarrhoea (2.2%) and no cases of colitis have yet been reported.
A dose-expansion study is ongoing evaluating the efficacy and safety of HMPL-689 in patients with relapsed/refractory lymphoma, said Cao.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.
