Welcome to ESMO 2021
Three trials, each with negative outcomes, were presented during a Proffered Paper session dedicated to head and neck cancers on Day 4 of the ESMO Congress 2021, highlighting the difficulties of introducing immunotherapy in this setting.
Limited advantages to avelumab use in LA-SCCHN
The phase 3 GORTEC-REACH trial (NCT02999087) showed mixed results when avelumab and cetuximab were added to radiotherapy and compared with standard of care in people with locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN). The experimental regimen provided some benefits among cisplatin unfit patients but was deemed futile among those who were cisplatin fit.
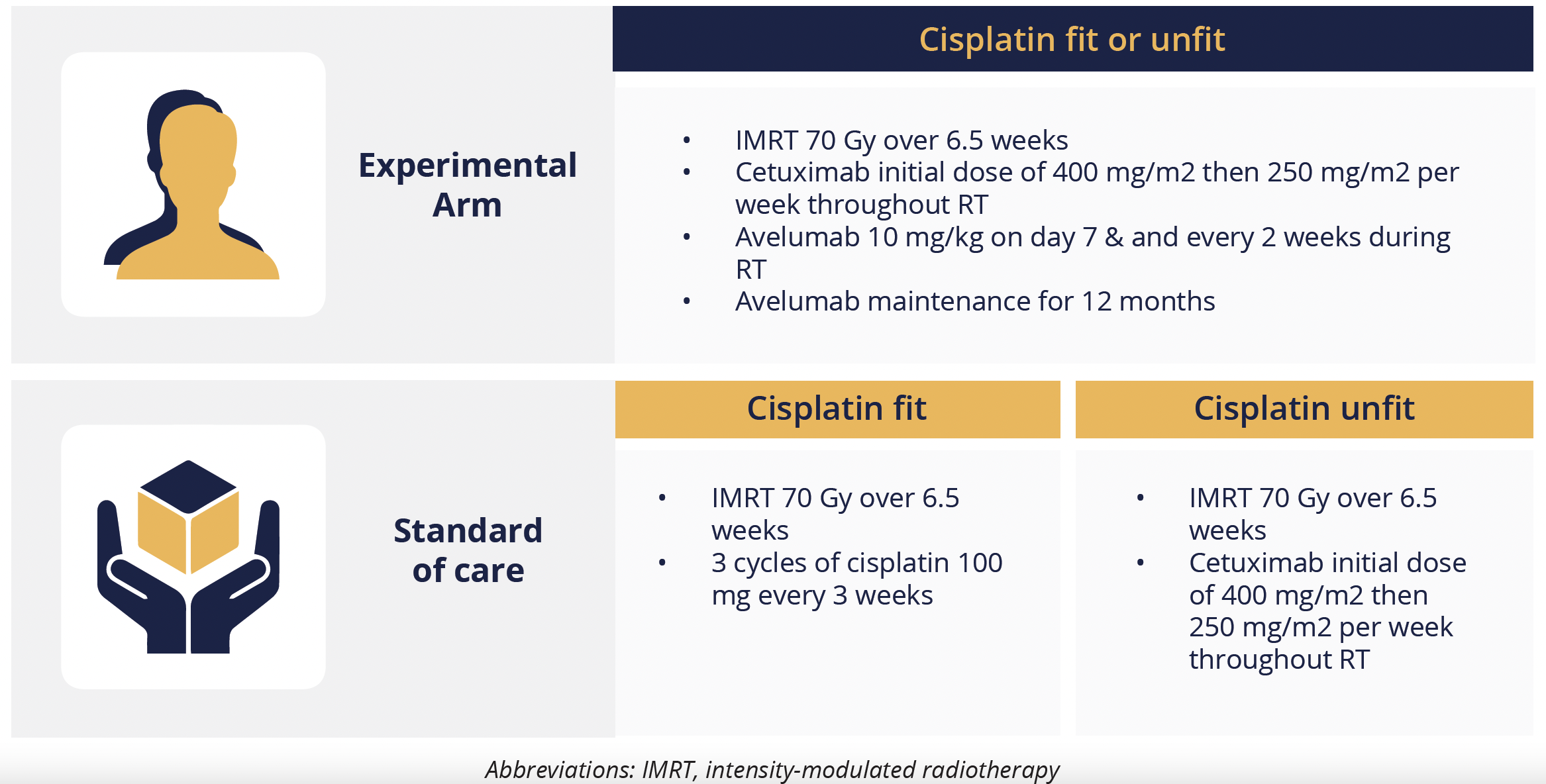
Treatment regimens for each arm
Jean Bourhis, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, reported that, among 277 cisplatin-unfit patients, the 2-year progression-free survival (PFS) rate was numerically, but not significantly, higher in the experimental arm relative to the standard of care arm, at 44% versus 31%.
There were also numerical improvements in the 2-year overall survival (OS) rate (58 vs 54%) and the 2-year locoregional progression rate (34 vs 44%), as well as a significant 69% reduction in the rate of distant metastases (5.4 vs 14.3%).
For the 430 cisplatin-fit patients, the required number of PFS events was not reached, and an interim futility analysis revealed that, at 1-year, the PFS rate was 64% in the experimental arm versus 73% in the cisplatin standard of care arm, with a nonsignificant hazard ratio of 1.27, which crossed the boundary for futility.
In both arms, 80% of participants experienced a grade 3 or worse adverse event (AE) most commonly dermatitis, mucocitis or dysphagia. There were 10 grade 5 AEs in the experimental arm, compared with three in the standard of care arm, but Bourhis noted that the participants were “fragile and old with numerous comorbidities” and their deaths were not a direct result of avelumab toxicity.
“The primary endpoint on PFS was not met.”
Jean Bourhis, Lausanne, Switzerland
No survival benefit with first-line nivolumab plus ipilimumab for platinum-ineligible RM-SCCHN
Final results of the phase 3 CheckMate 651 (NCT02741570) trial have shown that first-line nivolumab plus ipilimumab offers no significant survival benefit over the EXTREME regimen in patients with platinum-ineligible recurrent or metastatic squamous cell carcinoma of the head and neck (RM-SCCHN), but individuals with high PD-L1 expression may benefit from the immune checkpoint inhibitor combination.
Athanassios Argiris, from Hygeia Hospital in Athens, Greece, told delegates that, after a minimum 27.3 months of follow-up, median OS was 13.9 months among the 472 patients with RM-SCCHN who had received no prior systemic therapy for recurrent or metastatic disease and were randomly assigned to receive nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks.
This was not significantly different from the median OS of 13.5 months among the 475 participants who were given the EXTREME regimen of cetuximab plus cisplatin or carboplatin and fluorouracil for up to 6 cycles, followed by cetuximab maintenance until progression, unacceptable toxicity, or 2 years.
However, Argiris pointed out that there was a trend for improved survival among individuals with a PD-L1 combined positive score of 20 or more, with median OS at 17.6 months versus 14.6 months in the nivolumab plus ipilimumab and EXTREME arms, respectively.
He also noted that OS was better than expected in the EXTREME arm, which may be due to a high proportion (46%) of patients receiving subsequent immunotherapy. Just 8% in the nivolumab plus ipilimumab arm received subsequent therapy.
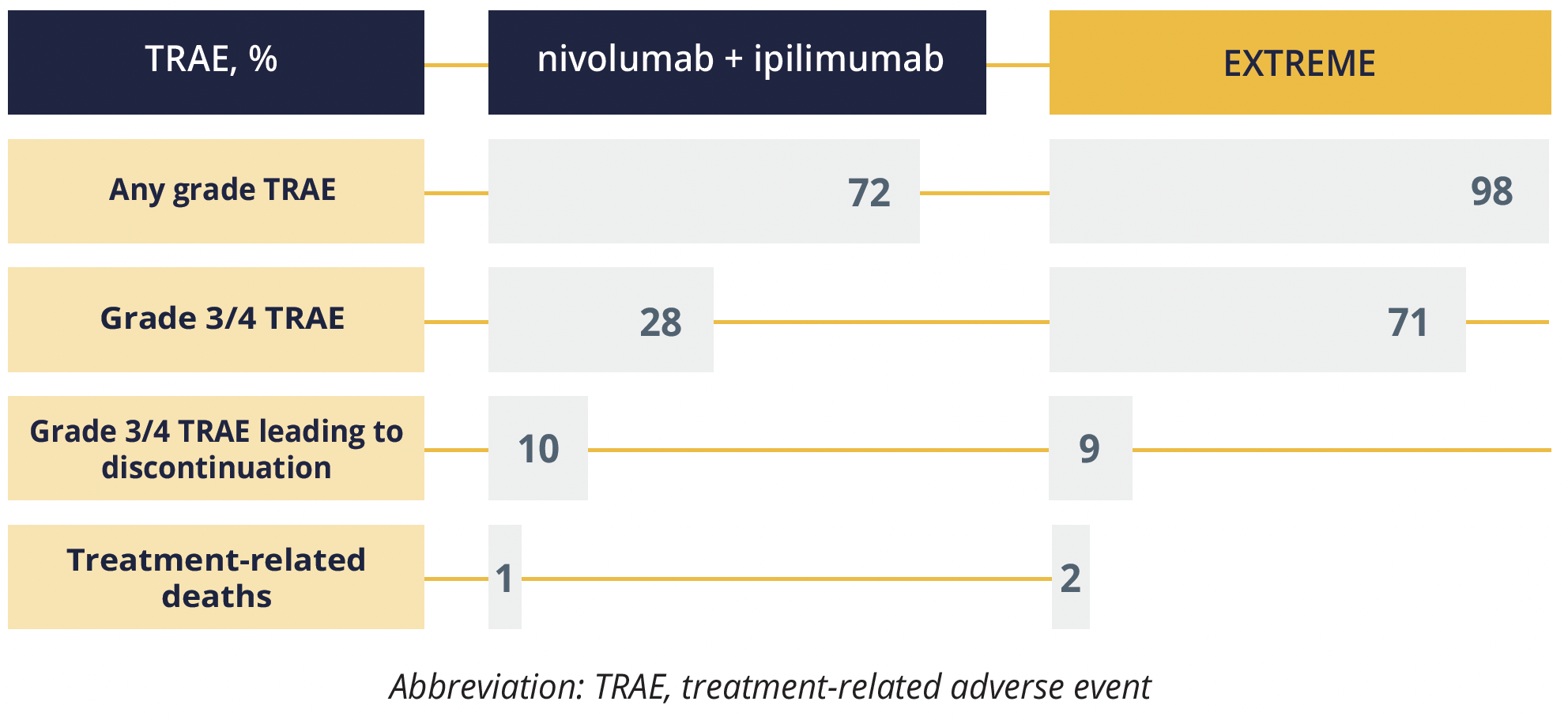
Treatment-related adverse events
Grade 3 or 4 treatment-related adverse events (AEs) occurred in fewer participants who received nivolumab plus ipilimumab, but discontinuation rates were similar to those among people who received EXTREME.
Discussant insights into GORTEC-REACH and CheckMate 651
During the session Amanda Psyrri, from the University of Athens in Greece, discussed the findings of the GORTEC-REACH and CheckMate 651 trials.

For GORTEC-REACH, she suggested that studies using enriched population-based strategies, such as PD-L1 expression, might identify a subpopulation who may derive benefit from the incorporation of PD-1 checkpoint inhibition into standard chemoradiation regimens.
Based on data from CheckMate 651 and other studies, Psyrri sad that “combined PD1 and CTLA-4 inhibition does not appear to be an effective strategy in RM-HNSCC, but future research may identify biomarkers to predict which patients are most likely to benefit.”
Pembrolizumab does not improve survival in platinum-pretreated RM-NPC
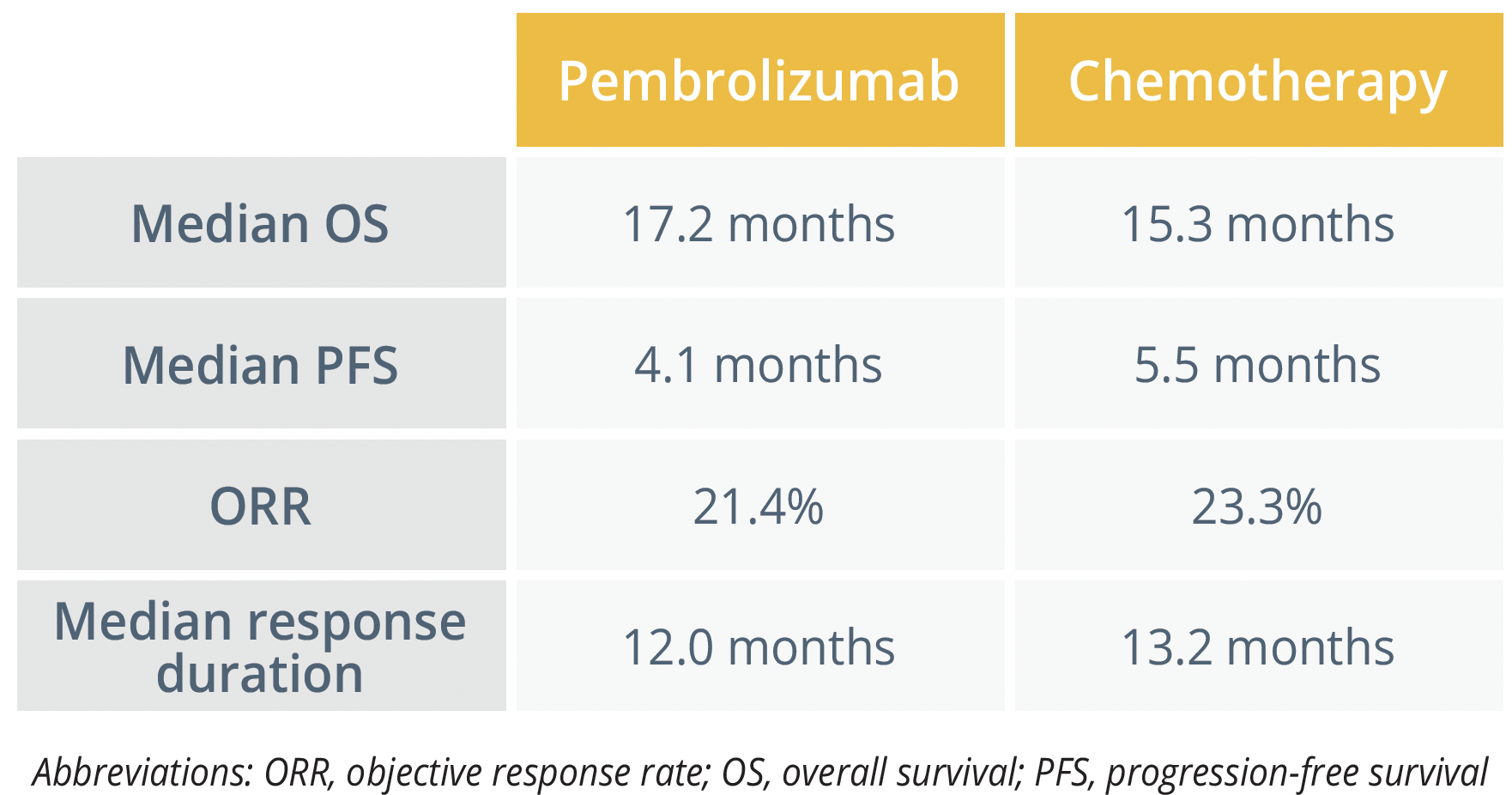
Anthony Chan, from The Chinese University of Hong Kong, China, reported that neither the primary outcome of OS, nor a number of secondary outcomes, were significantly improved with pembrolizumab monotherapy relative to chemotherapy for patients with recurrent or metastatic nasopharyngeal carcinoma (NPC) enrolled in the phase 3 KEYNOTE-122 (NCT02611960) trial.

The study included 233 participants who were randomly assigned to receive pembrolizumab 200 mg every 3 weeks for up to 35 cycles (n=117) or investigator’s choice of chemotherapy with capecitabine (n=39), gemcitabine (n=46) or docetaxel (n=31) at standard doses.
Despite the lack of improved efficacy, Chan said: “Pembrolizumab showed a manageable safety profile and a lower incidence of treatment-related adverse events versus chemotherapy.” The treatment-related AE rate of any grade was 61.2% with pembrolizumab and 87.5% with chemotherapy, with grade 3 and worse events occurring in a respective 10.3% and 43.8%.
“We still need to better understand the disease. NPC is a chemo sensitive cancer, it may be difficult to beat it with immunotherapy alone, especially in unselected patient populations.”
Lisa Licitra, Fondazione IRCCS Istituto Nazionale dei Tumori Milan, Italy
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.
