
Welcome to ESMO 2021
As the ESMO Congress 2021 draws to a close, we round up some of the key lung cancer research presented in the last couple of days, including two studies hoping to address the unmet treatment gap of non-small-cell lung cancer (NSCLC) with HER2 mutations, a Presidential symposium presentation providing additional insight into the IMpower010 trial and an update of the CASPIAN study in small-cell lung cancer (SCLC).
Targeting HER2 mutations in NSCLC
Two studies presented at the conference bring hope to newly diagnosed or previously treated patients with HER2-mutated NSCLC. Such mutations are found in around 2–4% of NSCLC cases and there are no approved HER2-targeted agents for this indication. But the DESTINY-Lung01 and ZENITH20 trials may be about to change that.
DESTINY-Lung01
The DESTINY-Lung01 study (NCT03505710) showed durable antitumour activity of the antibody–drug conjugate trastuzumab deruxtecan (T-DXd) in patients with inoperable or metastatic, nonsquamous, HER2-mutated NSCLC that is refractory to standard treatment.
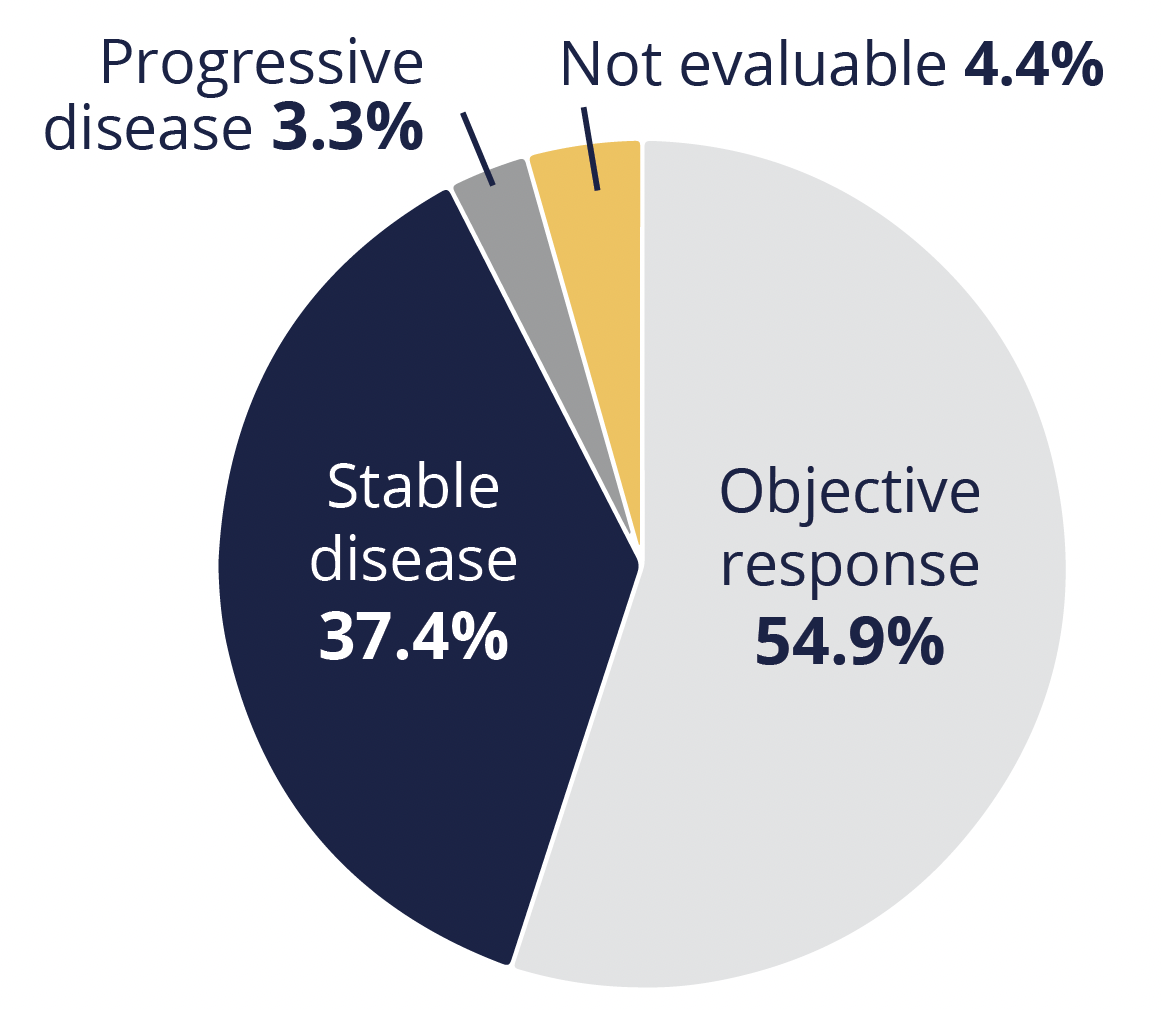
Presenting author Bob Li (Memorial Sloan Kettering Cancer Center, New York, USA) explained that the two-cohort phase 2 trial is evaluating the efficacy and safety of T-DXd in patients with HER2 mutations and in those with HER2 overexpression, with the current report focusing on the former group. At a median follow-up of 13.1 months, 54.9% of the 91 patients with HER2-mutated disease achieved an objective response, as assessed by independent review, following treatment with T-DXd 6.4 mg/kg every 3 weeks. A further 37.4% of the participants had stable disease, which meant that the disease control rate was 92.3%, and just 3.3% had progressive disease and 4.4% were not evaluable.
Disease outcomes
The median duration of response was 9.3 months, while the respective progression-free survival and overall survival (OS) times were 8.2 and 17.8 months.
Grade 3 or worse treatment-emergent adverse events related to the study drug occurred in 46.2% of patients, with neutropenia the most common event of this severity, at 18.7%. Li also provided data on the incidence of drug-related interstitial lung disease and pneumonitis, “an important identified risk.” Any-grade events occurred in 24 patients, with grade 3 cases in four and grade 5 in two.
“Overall, DESTINY-Lung01 provides compelling evidence of positive benefit–risk balance with T-DXd in the second-line or beyond settings, and supports its establishment as a potential new treatment standard for this patient population of unmet medical need”,
Bob Li, New York, USA
ZENITH20
Results from the multicohort ZENITH20 trial (NCT03318939) suggest that poziotinib, a pan-HER tyrosine kinase inhibitor (TKI), could be an option for patients with a new diagnosis of NSCLC with HER2 exon 20 insertion mutations.
Robin Cornelissen, from the University Medical Center Rotterdam in the Netherlands, presented data on 48 participants from cohort 4 of the study, all of whom had treatment-naïve disease and who received poziotinib at a dose of 16 mg/day.
Disease outcomes
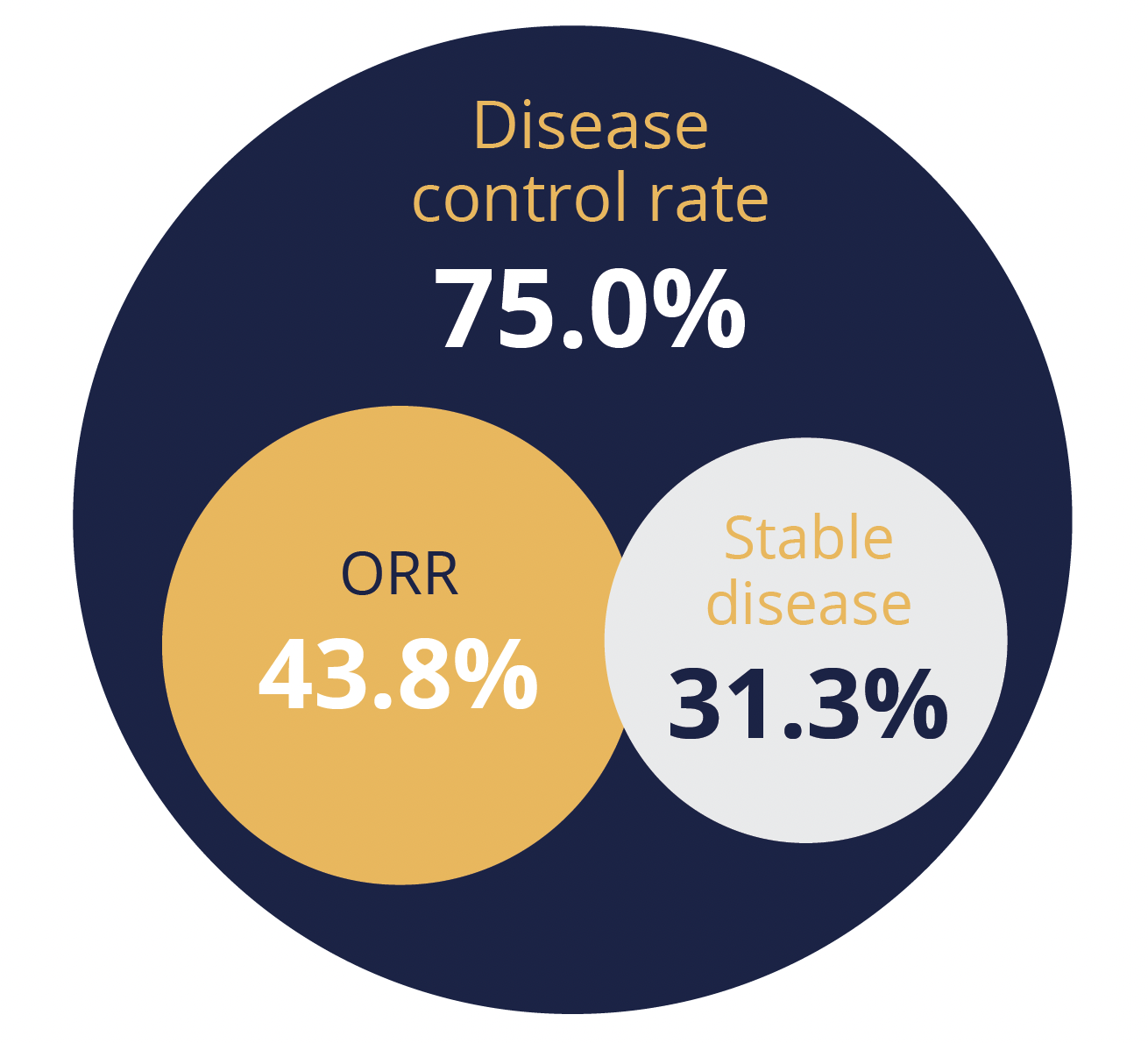
Over a median follow-up of 13.5 months, the independently-assessed objective response rate was 43.8%, with responses lasting for a median of 5.4 months. A total of 31.3% of participants achieved disease stabilisation, giving a disease control rate of 75.0%, while 14.6% had progressive disease and 10.4% were unevaluable.
Poziotinib had a “manageable” toxicity profile that was in line with previous studies of the agent and other second-generation EGFR–TKIs, said Cornelissen. There were no adverse events of grade 4 or 5, and the most common events of grade 3 were rash, stomatitis or mucosal inflammation, and diarrhoea, in 35%, 21% and 15% of patients, respectively. And just one patient experienced grade 3 pneumonitis.
Dose interruption and reductions were required by a respective 88% and 77% of participants, but only 13% permanently discontinued study treatment due to toxicity.
The presenter therefore concluded that “poziotinib shows clinically meaningful efficacy” in this patient population, adding that cohort 4 continues to enrol patients who are receiving poziotinib at a dose of 8 mg twice daily, which he hopes will further improve tolerability and efficacy.
Sites of relapse and post-relapse treatment in IMpower010
Delegates attending the third Presidential symposium heard about additional data from the phase 3 IMpower010 trial (NCT02486718) of adjuvant atezolizumab in early-stage NSCLC, specifically about the patterns of relapse and post-relapse therapies.
The findings were presented by Enriqueta Felip, from Vall d’Hebron Institute of Oncology in Barcelona, Spain, and discussed by Benjamin Besse, from Institut Gustave Roussy in Villejuif, France.
The trial recruited 1005 patients with completely resected stage IB–IIIA disease who had received one to four cycles of adjuvant platinum-based chemotherapy, and randomly allocated them to receive up to 16 cycles of atezolizumab 1200 mg every 3 weeks or best supportive care (BSC) after chemotherapy.
Disease relapse rates in different treatment populations
As reported earlier this year, the use of adjuvant atezolizumab was associated with a significant disease-free survival (DFS) benefit relative to BSC in stage II–IIIA patients with PD-L1 positivity (tumour cell expression ≥1%) and in all patients with stage II–IIIA disease, with hazard ratios of 0.66 and 0.79, respectively. Atezolizumab was also favoured in the intention-to-treat (ITT) population that included all patients with stage IB–IIIA NSCLC, albeit without reaching statistical significance at the time of analysis.
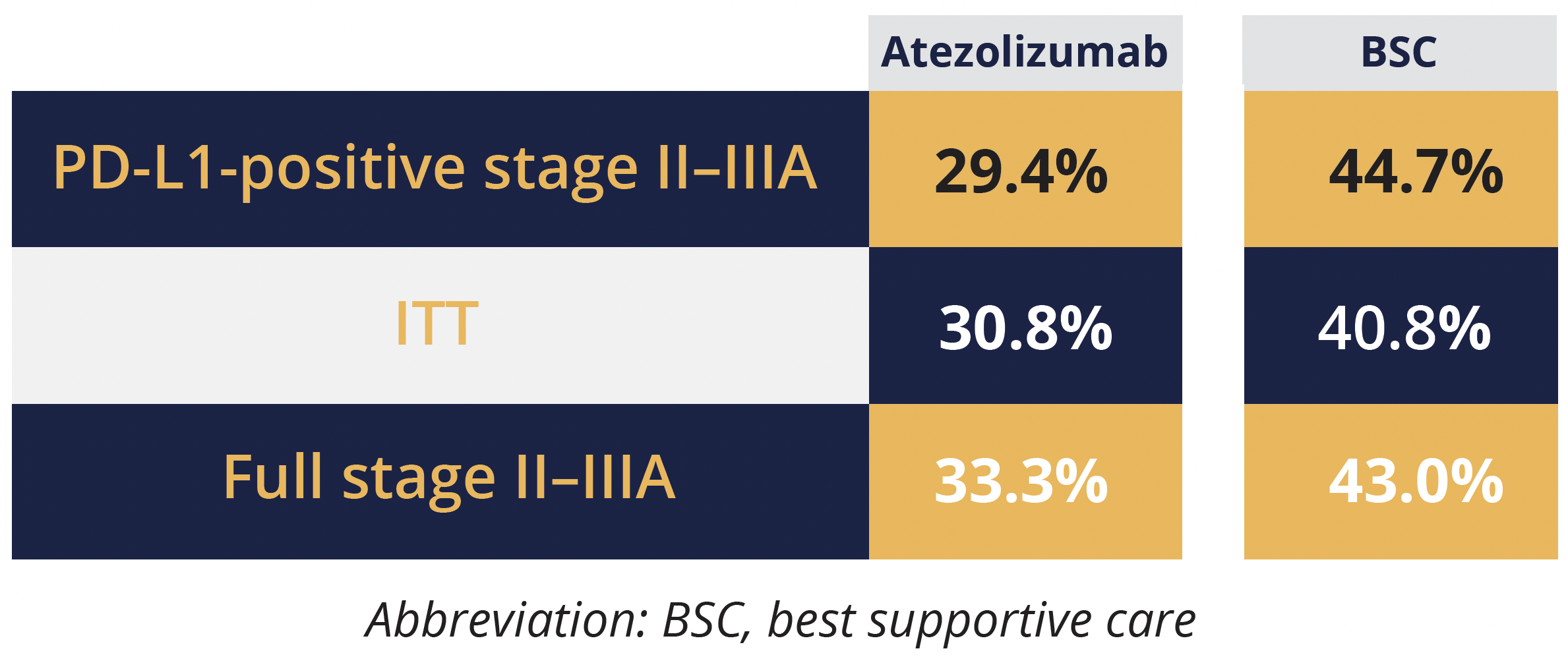
In the current presentation, Felip focused on exploratory analyses of disease relapse, the rates of which tended to be lower with atezolizumab than BSC in all three populations, at 29.4% versus 44.7% in the PD-L1-positive stage II–IIIA population, 33.3% versus 43.0% in the full stage II–IIIA population, and 30.8% versus 40.8% in the ITT population.
However, there were “no clear differences in the patterns of relapse” between the two treatment arms when analysed by relapse site, namely locoregional or distant only, locoregional and distant, and central nervous system only, Felip told the audience. She also shared data on post-relapse systemic and local treatments in those with disease recurrence, noting that around 65% of patients received systemic therapies, with the rates and types of therapies generally well balanced between treatment arms and across the study populations.
The discussant highlighted, however, that post-relapse immunotherapy was only used in around a third of control patients, which indicates a “low rate of cross-over” and is “a potential concern”, given that immunotherapy – either alone or together with chemotherapy – is a standard of care in the first-line.
He also drew attention to the “surprisingly” high rates of local treatments – around 15% of patients underwent surgery and over a third received radiotherapy – which could suggest that the original resection and preoperative work-up were not optimal.
Besse believes that questions remain to be answered, such as the optimal population to receive neoadjuvant atezolizumab as there did not appear to be a benefit in patients with PD-L1 levels below 1%.

Updated CASPIAN data show continued benefit of durvalumab–chemo in extensive-stage SCLC
Extended follow-up of the CASPIAN trial (NCT03043872) continues to support the use of durvalumab alongside first-line chemotherapy in patients with extensive-stage SCLC.
Nearly three time as many patients in the durvalumab plus chemotherapy arm were alive at the 3-year timepoint as those in the chemotherapy alone arm, at 17.6% versus 5.8%, reported study author Luis Paz-Ares (Hospital 12 de Octubre, Madrid, Spain).
And after a median 39.4 months of follow-up, the risk of death was a significant 29% lower with the addition of durvalumab, he added.
These results follow on from the primary analysis that showed a significant 27% reduction in the risk of death among the 268 participants who were randomly allocated to receive four cycles of durvalumab 1500 mg alongside etoposide–platinum every 3 weeks versus the 269 who received up to six cycles of chemotherapy alone.
The current analysis also showed a numerical improvement in 3-year OS with the addition of durvalumab and tremelimumab to chemotherapy, at a rate of 15.3% compared with 5.8% for chemotherapy alone, reported Paz-Ares.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.
