
Welcome to ESMO 2021
The opening day of the ESMO Congress 2021 saw exciting research presented in the lung and other thoracic cancers space, ranging from a trial update confirming the role of immunotherapy for the treatment of mesothelioma to an investigation of novel durvalumab combinations in locally advanced non-small-cell lung cancer (NSCLC).
CheckMate 743 update supports nivolumab–ipilimumab use in mesothelioma
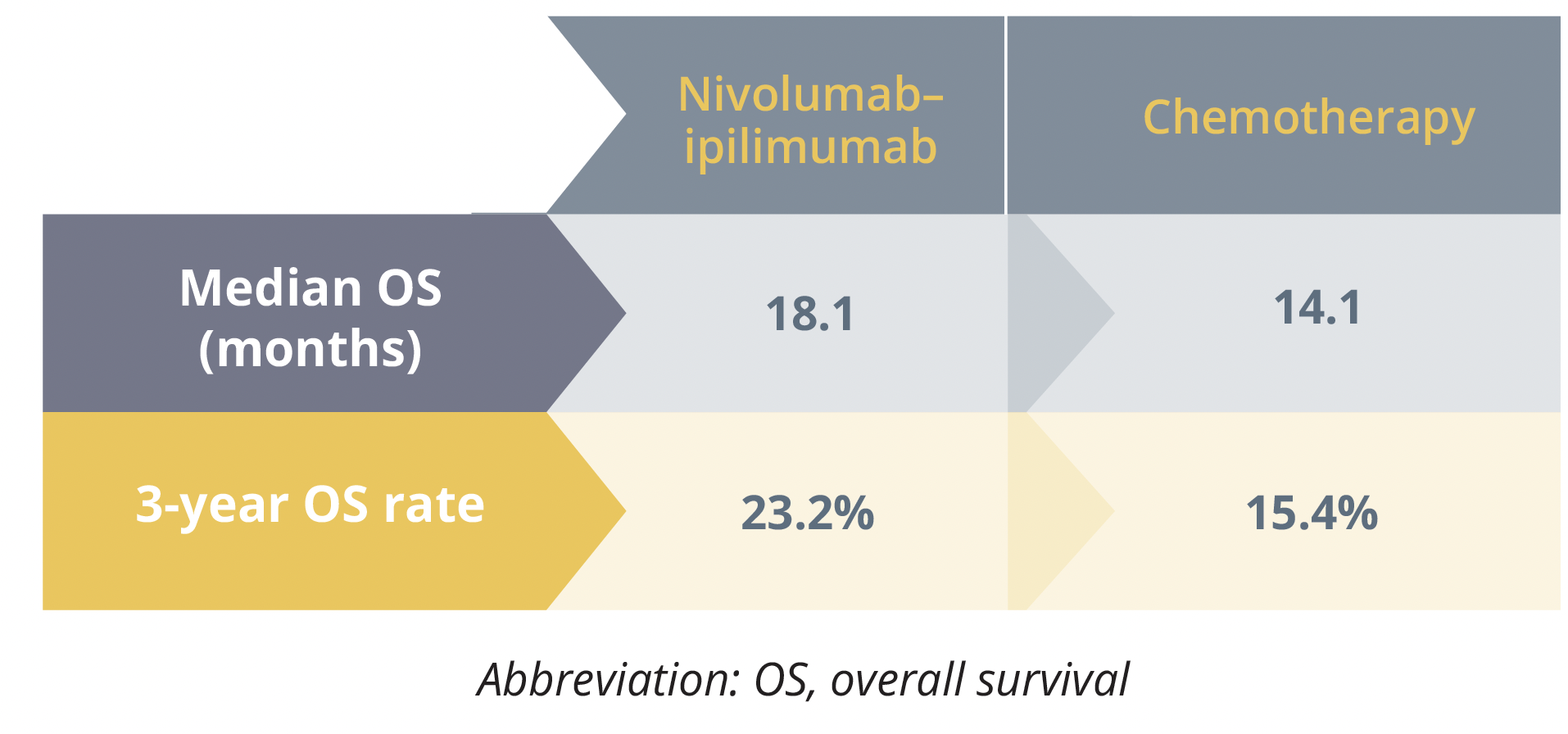
The 3-year data from the CheckMate 743 trial (NCT02899299) show that the overall survival (OS) benefit offered by dual checkpoint blockade in treatment-naïve patients with inoperable malignant pleural mesothelioma is maintained over time.
The study investigators previously reported that the risk of death was 26% lower for the 303 participants who were randomly assigned to receive nivolumab plus ipilimumab for up to 2 years than the 302 who received six cycles of cisplatin or carboplatin alongside pemetrexed.
Overall survival results
The current analysis, conducted at a minimum follow-up of 35.5 months, mirrored the earlier findings and showed a significant 27% reduction in the risk for death with combination immunotherapy relative to chemotherapy.
Presenting author Solange Peters (Lausanne University Hospital, Switzerland) also highlighted that “[w]ith 12 additional months of follow-up, safety was consistent with the previous report, with no change in the overall rate of TRAEs [treatment-related adverse events].”
Specifically, TRAEs of grade 3–4 occurred in a comparable 31% of patients treated with nivolumab plus ipilimumab and 32% of those given chemotherapy, but the rate of discontinuation of any component due to TRAEs was higher in the combination immunotherapy than chemotherapy arm, at 15% versus 7%.
“These results confirm nivolumab plus ipilimumab as a standard of care in this patient population”
Solange Peters, Switzerland
Combining durvalumab with novel agents shows promise in locally advanced NSCLC
Phase 2 COAST study findings (NCT03822351) point to the potential of combining durvalumab with the anti-CD73 agent oleclumab or the anti-NKG2A agent monalizumab as consolidation therapy in patients with stage III NSCLC who have not progressed after chemoradiotherapy.
The presenter – Alexandre Martinez-Marti, from Vall d’Hebron Institute of Oncology in Barcelona, Spain – explained that radiotherapy has been shown to induce the expression of CD73 and NKG2A ligand, “which inhibit antitumour immune response.”
The investigators therefore randomly assigned 186 patients to receive consolidation durvalumab 1500 mg every 4 weeks, either alone or alongside intravenous oleclumab 3000 mg (every 2 weeks for first two cycles and every 4 weeks thereafter) or intravenous monalizumab 750 mg every 2 weeks.
The investigator-assessed objective response rate was higher in both the oleclumab and monalizumab combination groups than the durvalumab alone group, at 30.0% and 35.5% versus 17.9%, respectively.
This was also the case for disease control, with corresponding rates at 16 weeks of 81.7%, 77.4% and 58.2%, and progression-free survival, with the median times unreached, of 15.1 months and 6.3 months, respectively.
This boost in outcomes did not appear to come at the cost of toxicity – the incidence of grade 3 or worse AEs was similar between the oleclumab and monalizumab combination and durvalumab alone arms, at 40.7%, 27.9% and 39.4%, respectively, as were the rates of serious TRAEs, at 11.9%, 8.2% and 9.1%.
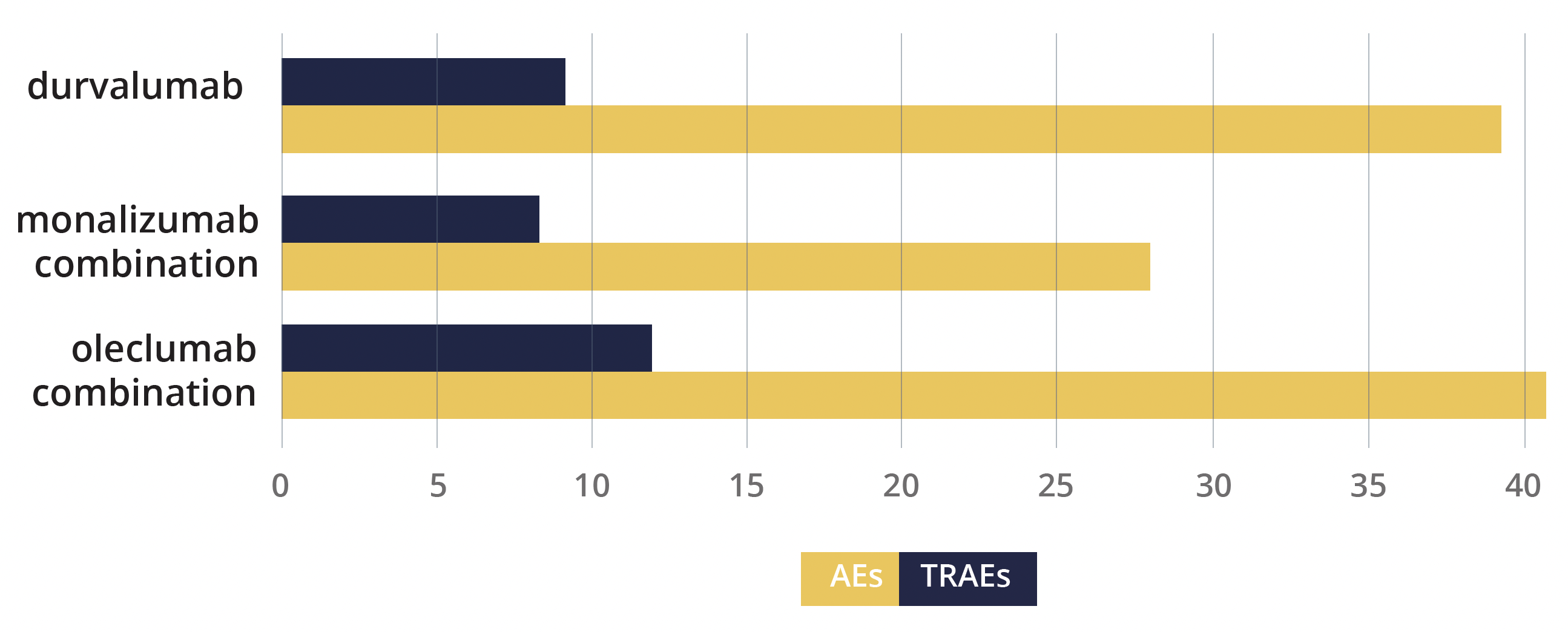
Adverse events and serious treatment-related adverse events
“These data support further evaluation of these combinations in a registration-intent study”
Martinez-Marti, Barcelona, Spain
Virtual plenary debate
The day also saw debates and discussions of two studies reported at an ESMO Virtual Plenary earlier in the year. The first debate focused on the phase 2 ETOP BOOSTER trial (NCT03133546) and involved investigator Ross Soo, from the National University Hospital in Singapore, and discussant Pasi Jänne, from the Dana-Farber Cancer Institute in Boston, Massachusetts, USA.
The study – comprising 155 participants – found that adding bevacizumab to second-line osimertinib did not significantly improve the outcomes of people with advanced NSCLC positive for activating EGFR mutations and acquired T790M mutations.
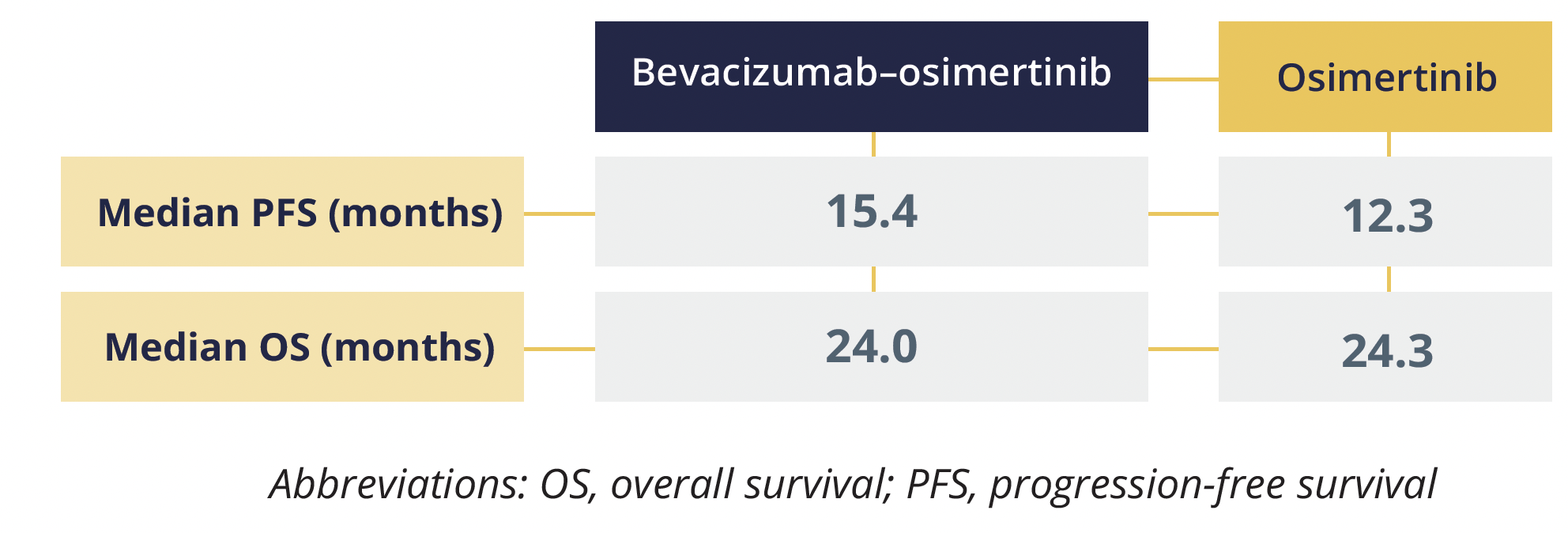
Progression-free and overall survival data
The debate addressed questions such as why only the studies combining an antiangiogenic agent with erlotinib seem to be positive, whether any clinical situations warrant the first-line use of erlotinib alongside bevacizumab or ramucirumab rather than osimertinib alone, and what the future holds for the combination of antiangiogenic agents and EGFR–tyrosine kinase inhibitors.
The second debate involved study author Solange Peters (Lausanne University Hospital, Switzerland) and discussant Marina Garassino (University of Chicago, Illinois, USA). They talked about a real-world database analysis comprising 520 patients with metastatic nonsquamous NSCLC and high PD-L1 expression (tumour proportion score ≥50%), the results of which suggest that first-line anti-PD-1 or anti-PD-L1 monotherapy is sufficient for most patients (except perhaps never smokers) and platinum-based chemotherapy can be omitted.
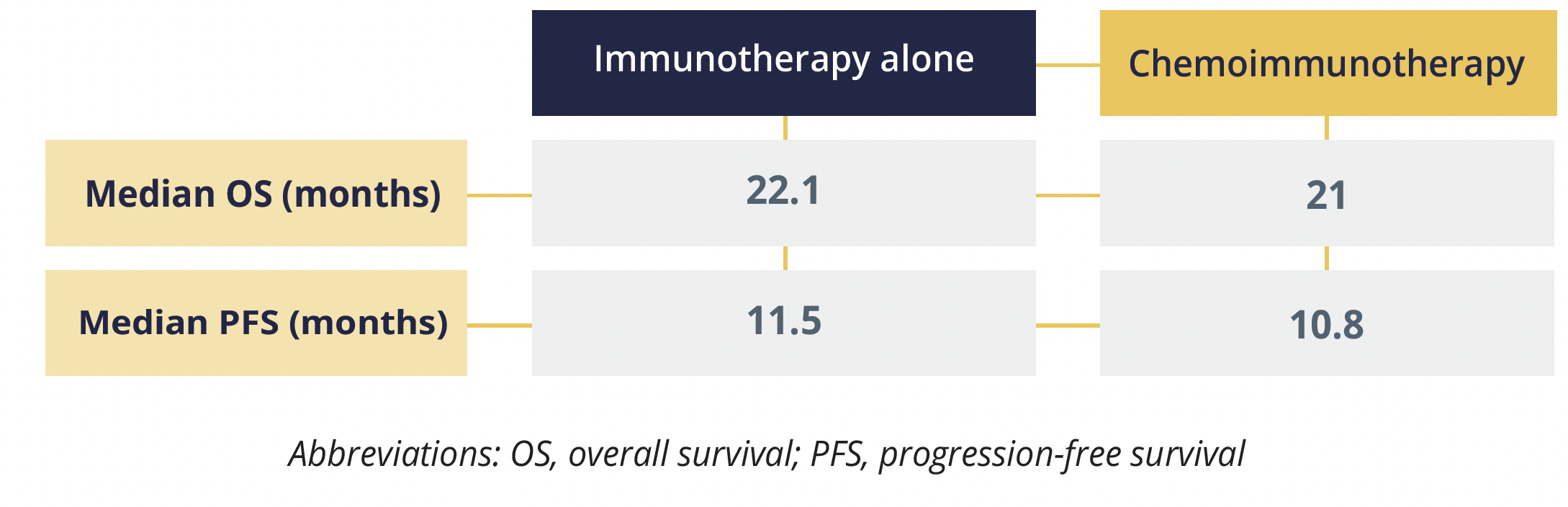
Progression-free and overall survival data
The main discussion points in this debate were the limited sample size and attrition, the discrepancies between randomized controlled trials and real-world studies, and the need for subgroup analyses to help elucidate the best treatment option for women, older patients (>75 years) and those with very-high PD-L1 expression levels (>90%).
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. This content is not intended for use by healthcare professionals in the UK, US or Australia. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.
