
Welcome to ESMO 2022
-
The Annual Meeting of the European Society of Medical Oncology (ESMO) was a hybrid meeting, with delegates attending in person for the first time since before the pandemic. ESMO President Solange Peters noted that this made the meeting “feel like a huge success before it has even begun” as the oncology community were able to meet, network and disseminate data in-person in Paris, France. She underlined that, for ESMO, there was never going to be a return to “normal” but was instead progressing forward due to the proactivity that the community has demonstrated in reinventing itself to meet the challenges of our era.

New cancer cases predicted globally by 2030 and 2040
Globally, the ESMO community is already taking action by going the extra mile to comfort patients, to grow as professionals by keeping their knowledge up-to-date, and by raising awareness for cancer prevention. The ESMO community is driven by a shared determination to work towards the best possible outcomes for patients with an inspiring tagline of, ‘Together we can. Together we care.’
Solange Peters then summarised the importance of healthcare sustainability and how our healthcare systems rely on finite resources. She emphasised that a key responsibility of the oncology community should be safeguarding patients’ ability to access high-quality care. ESMO should continue to contribute to the overall sustainability of the healthcare system by nurturing the professional development of oncologists and offering continued support in their day-to-day clinical practice. ESMO’s support and resources should therefore reach every oncology professional.
“As important as doctors’ knowledge and skill is their physical and mental ability to give the best of themselves to patients each day.”
- Solange Peters, ESMO President
Solange Peters then introduced the ESMO 2022 Scientific Co-Chairs, Fabrice André and Charles Swanton. Charles Swanton gave an overview of the impressive proportion of delegates attending ESMO 2022 both in-person and online. He then stated that the purpose of ESMO 2022 is not only a “celebration of getting us all back together”, but also a collaboration between diverse oncology professionals who spend their lives aiming to improving the survival of their patients. ESMO 2022 will focus on “better understanding the disease and treating patients better – from bench to bedside”, which is a matter of time, collaboration, patience and long-term investment. Fabrice André added that current challenges in oncology will be integrated within the ESMO Congress 2022 programme such as early cancer detection and prevention, molecular medicine, and the impact of new drugs assessed in underrepresented patients such as anti-programmed cell death protein-1 (PD1) in the elderly population.

Total participants online and in person
Aetiology
As mentioned by ESMO President Solange Peters in the Opening Session of ESMO 2022, the link between smoking, alcohol consumption and cancer has been extensively described, but our understanding of the impact of environmental factors such as air pollution is still evolving. ESMO 2022 Scientific Co-Chair, Charles Swanton, London, UK, gave an overview of the relationship between air pollution and non-small cell lung cancer (NSCLC) in this pivotal late-breaking abstract summary. He highlighted that this proposed association is not a new theory and the risk between air pollution concentration and lung cancer been suspected for over two decades. What is not yet known, however, is a specific causation. Lung cancer in never-smokers is a disease with a low mutational burden and patients do not have a clear environmental carcinogenic signature.

Criteria for environmental pollution to be considered a cause of lung cancer
He discussed whether air pollution could act as a lung cancer promoter as PM2.5 pollution levels (e.g. from diesel exhausts and fossil fuels) could cause tumourigenesis of pre-existing EGFR-mutant clones in murine models. In addition, he proposed that initiator cells exist with EGFR driver mutations in normal human lung tissue, thus supporting the classic mutation model by Berenblum developed in 1947. Firstly, UK biobank data from 447,932 participants were assessed and found that rising PM2.5 levels were associated with an increase in incidence of seven cancer types, including lung, head and neck, and gastrointestinal cancers. In addition, a significant association (p=00077; R=0.58) was found between 2018 regional EGFR-mutant NSCLC incidence per 100,000 patients in England with rising mean PM2.5 concentrations (μg/m3).
This analysis was repeated in South Korea and Taiwan where higher levels of PM2.5 have been observed compared with England and the relationship between EGFR-mutant global lung cancer incidence was still found.
Causation was assessed in a murine model whereby an EGFR or KRAS mutation was induced at Day 0 and then the mice were exposed to intra-tracheal PM2.5 from Day 3 onwards (every 2 days for 3 weeks). At Week 10, the mouse lungs were assessed for lung tumours and a dose-dependent(5 and 50 μg PM2.5) increase in tumours was observed. The researchers concluded that exposure to PM2.5 induced an AT2 progenitor state transcriptional signature and macrophage recruitment in these murine models. However, neither air pollution nor EGFR mutations alone were deemed to be sufficient to augment a stem cell state, so stem cell capacity could require the mutation as an initiator in addition to air pollution as a promoter.
The transcriptional response to PM2.5 was compared in human lung tissue versus the mouse lung to determine the key cytokines involved. Interleukin-1β (IL-1β) was identified as the most commonly upregulated gene in both species, which induced an alveolar type 2 progenitor state. Prof Swanton then related these findings to the CANTOS trial by Ridker et al. (2017) that found that administration of 150 or 300 mg of canakinumab, an IL-1β inhibitor, to patients with atherosclerosis was associated with reduced lung cancer incidence. He then outlined the results of a further study conducted in mice whereby they were exposed to PM2.5 simultaneously with an anti-IL-1β antibody (8 mg/kg) three times per week versus control. Blocking IL-1β was shown to inhibit air pollution-induced cancer in this EGFR-mutant mouse model.
He concluded that we are one step closer to understanding the link between PM2.5 exposure and EGFR-mutant lung cancer, and why there may be an absence of a mutagenic signature associated with air pollution. He hoped this could open more doors in molecular cancer prevention in high-risk patient populations.

Potential theories of carcinogenic properties of UV light, tobacco and vaping
The findings of this study also raise more questions on whether other environmental exposures such as UV light and tobacco could act as both an initiator and promoter through mutations. He feared that this could raise some concerns about the safety of vaping as there is currently no long-term follow-up data on its effects on lung tissue and could promote cancer in a similar mechanism to air pollution exposure.
“We have no choice over the air we breathe and the poorest areas are more exposed to the highest levels of PM2.5s, so how do we solve this societal problem?"
- Charles Swanton, London, UK
Suzette Delaloge, Villejuif, France, discussed this late-breaking abstract and outlined the implications of these data on public health. First and foremost, she highlighted that we must reduce exposure to PM2.5 air pollution worldwide. She suggested that the findings could act as a model for cancer interception, which is a combination of early detection and early biomarker-driven prevention therapies to eradicate cancers before their clinical phase.
Cancer interception
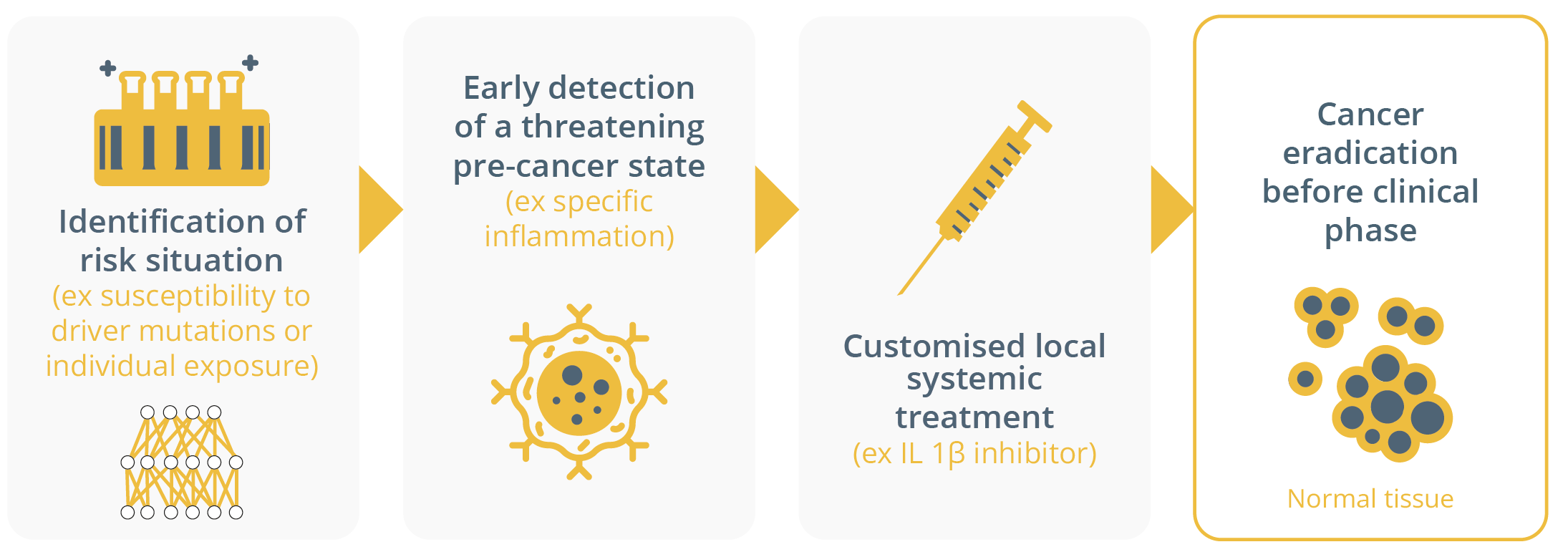
A model for cancer interception
Taken together, these data and the CANTOS trial results could provide an alternative “non-mutagenic carcinogenic promotion hypothesis” for fine particulate matter, as shown below for normal lung cells bearing EGFR mutations exposed to air pollution.
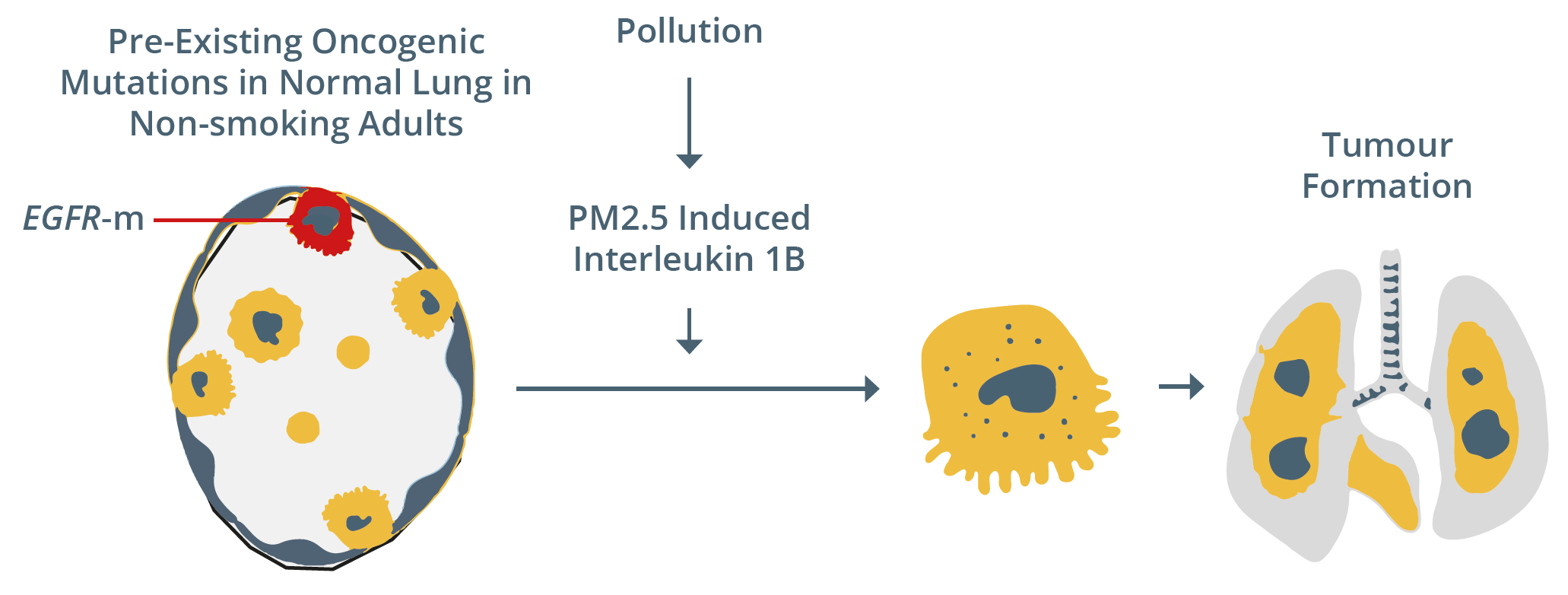
Potential mechanism behind tumour formation following exposure to PM2.5
The findings of this late-breaking abstract were influential enough to be selected for discussion in a Congress Highlights session. Masahiro Tsuboi, Yokohama, Japan, summarised that this key study shed an interesting light on the aetiology of EGFR mutant lung cancer, particularly in never-smokers.
Treatment
Immunotherapy
Solange Peters discussed how the efficacy of immunotherapy in lung cancer could change based on decisions surrounding treatment duration, intervals and dosage. She outlined that the concept of immunotherapy is based on the ‘four pillars of promise’ to optimise the immunological response for durable tumour control and ultimately aim for curative treatments.

Four pillars of promise to optimise immunological response for durable tumour control
She then gave an overview of the growing landscape of immunotherapy drugs and how the hallmarks of combination therapy should be long-term benefit, depth and duration of response, treatment-free survival, safety and quality of life (QoL). Long-term benefit is a key aim of immunotherapy and has been assessed in the CheckMate 003 (assessing 6-year overall survival [OS] following nivolumab treatment), CheckMate 017/057 (assessing 4-year OS following nivolumab treatment) and Keynote-001 (assessing 5-year OS following pembrolizumab treatment). She pointed out that the immunotherapy approaches applied in these studies were associated with a ‘tail of the curve’. She suggested that these results pose important questions such as, “How much immunotherapy do we need? And for how long?” to achieve long-term benefit.
Hallmarks of combination immunotherapy treatment
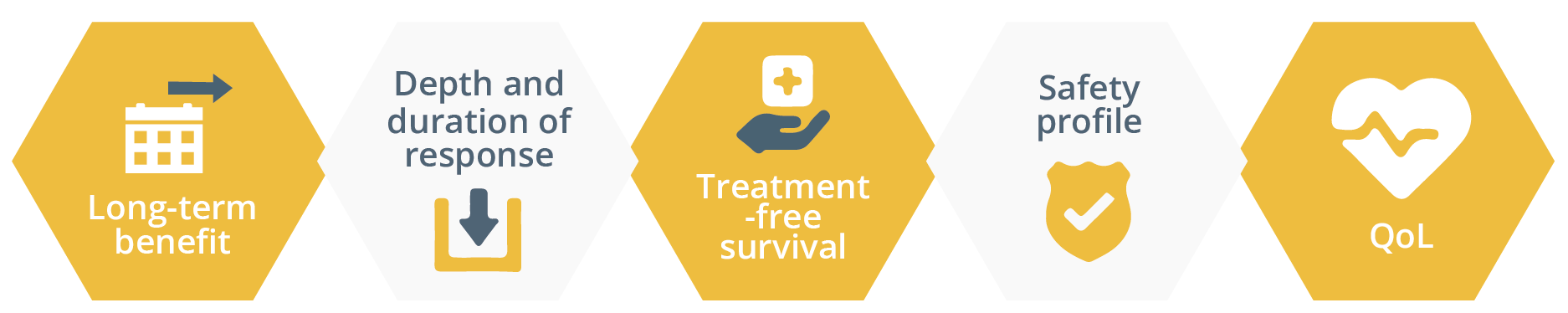
Hallmarks of combination immunotherapy treatment
Prof. Peters evaluated some immunotherapies and pointed out that even though that they are distinct molecules, they have similar ‘clinical impact’ in NSCLC. She highlighted that each IgG isotype has a distinct binding affinity to the various Fc receptors (FcyRs) and reminded delegates that immunotherapy drugs also differ in their level of PD-1 occupancy, volume of distribution, toxicity profile and serum half-life. A combination of these differing features can lead to diverse and highly regulated antibody responses.
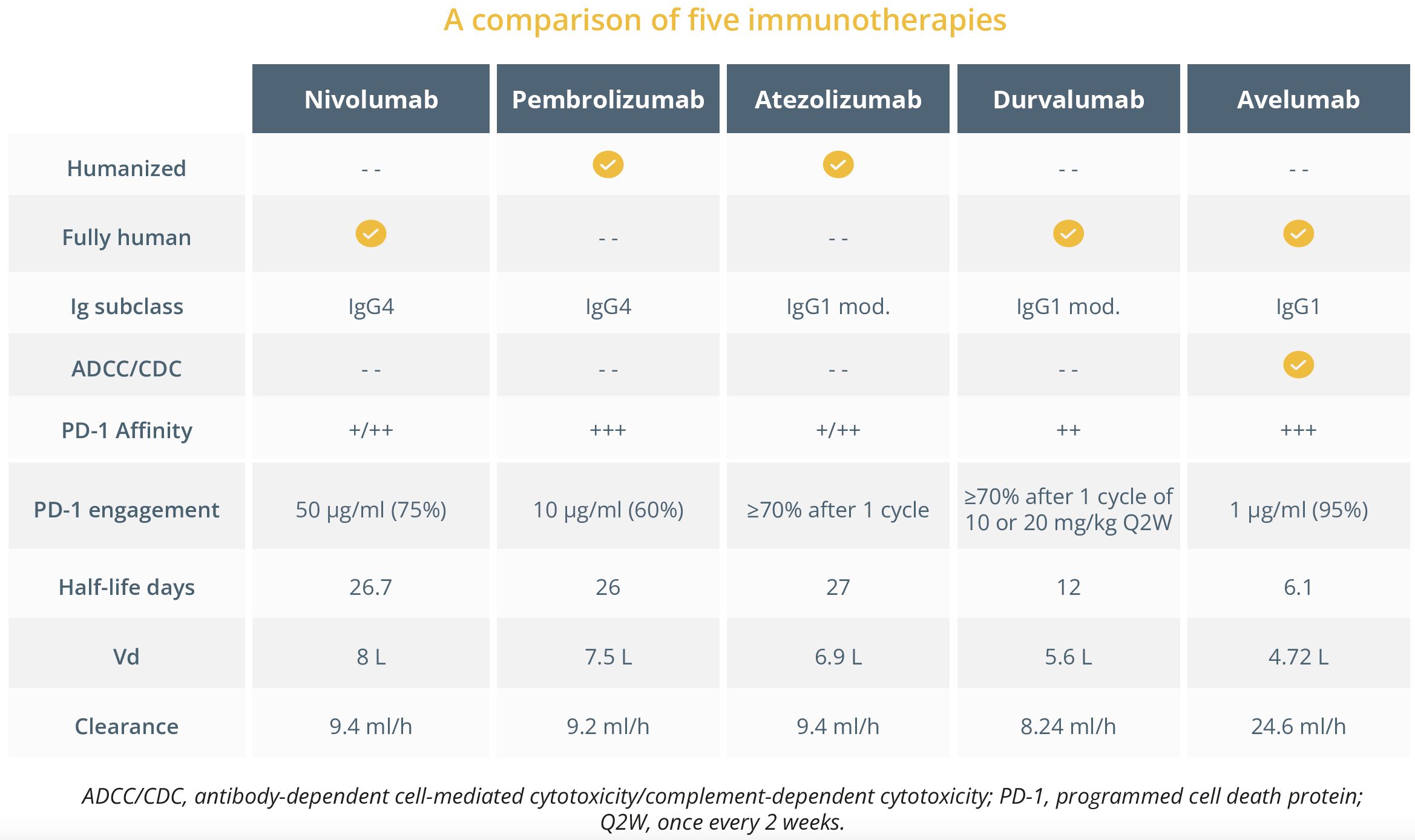
A comparison of five immunotherapies
She then gave an overview of anti-drug antibody (ADA) levels observed in the key, pivotal NSCLC trials of OAK (atezolizumab versus docetaxel [N=565]), IMpower010 (adjuvant atezolizumab after adjuvant chemotherapy [N=487]), and IMpower150 (atezolizumab plus bevacizumab and chemo-therapy [N=634]). ADAs were 30%, 31%, and 36% in the OAK (median onset time to ADA formation was 3 weeks), IMpower010, and IMpower150 trials, respectively.
She then compared the approaches of ongoing clinical trials assessing immunotherapy in resectable NSCLC. Despite the differing paradigms of these trials, she suggested that if immunotherapy is continued beyond induction, the consolidation treatment duration would generally be one year and questioned why this is usually the case with some exceptions.
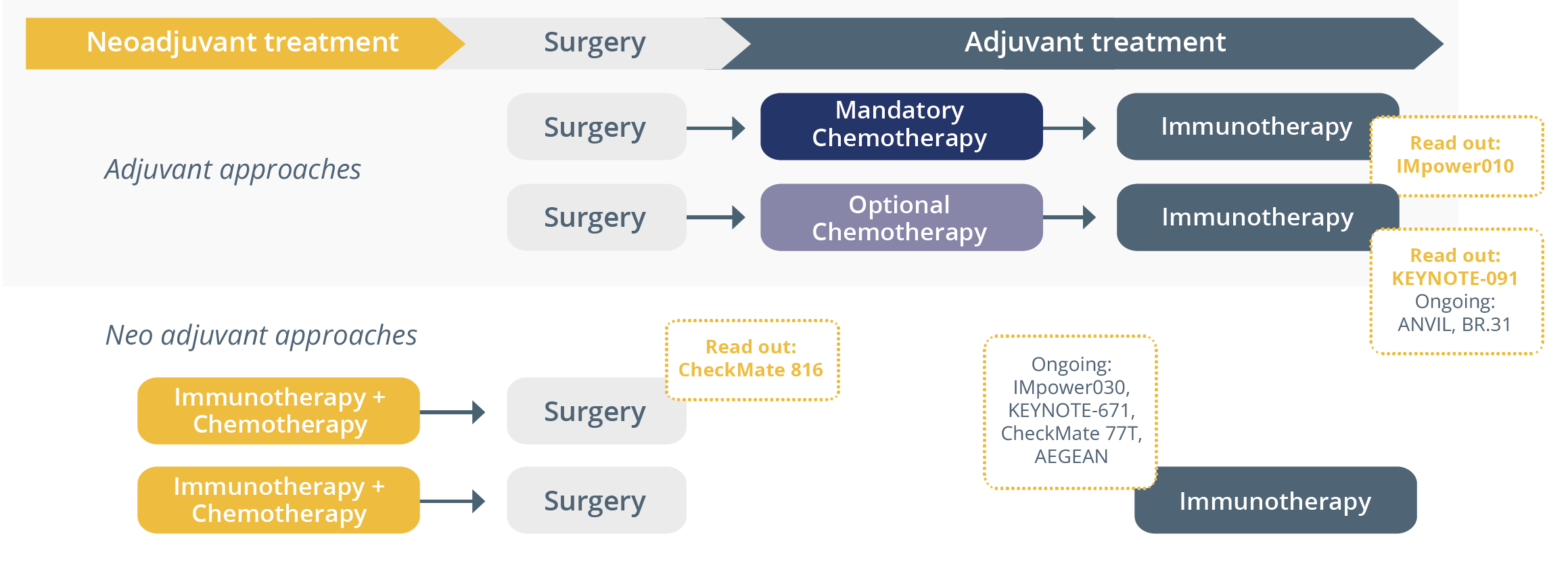
Study designs of ongoing clinical trials of immunotherapy in resectable NSCLC
Targeting minimal residual disease (MRD) in NSCLC
MRD is defined as the small volume of tumour cells remaining after treatment in patients who have no clinical evidence of disease. Maximilian Diehn, Stanford, USA, stressed the importance of detecting MRD shortly after completion of local therapy for patients with lung cancer and how this could ultimately allow for personalisation of adjuvant treatment. He stated that circulating tumour DNA (ctDNA) is a promising tool to address the unmet need of measuring MRD across tumour types. ctDNA has been shown to act as a strong prognostic biomarker for MRD regardless of NSCLC stage, treatment and ctDNA assay used. However, a key limitation of first generation ctDNA MRD assays is a high false negative rate and this should be considered. He outlined how ctDNA MRD analysis was applied in the IMpower010 trial which demonstrated that the benefit of consolidation therapy was strongest in ctDNA-positive patients compared with ctDNA-negative patients, but pointed out that the latter still also appear to benefit, suggesting that existing ctDNA MRD assays are not sufficiently sensitive yet and this needs to be addressed. He suggested based on recent data that sensitivity below 0.01% is needed to decrease false negative rates. He proposed that PhasED-Seq could improve sensitivity for ctDNA MRD detection and may ultimately allow for personalised adjuvant therapy, but underlined that prospective trials in NSCLC are needed to confirm this. ESMO President Solange Peters later added that therapy de-escalation for MRD is not appropriate due to the false negative rate of current ctDNA MRD assays.
“While specificity issues (of ctDNA MRD assays) might be overcome by personalised tissue-based testing, sensitivity remains the bottleneck,”
- Solange Peters, ESMO President
Closing Remarks
Following a successful in-person meeting this year with ~23,000 attendees, ESMO 2023 will continue to disseminate the latest cutting-edge data and provide a unique networking opportunity for oncology professionals in-person in Madrid, Spain, from 20–24 October 2023. Save the date for what will be an equally engaging and successful meeting in 2023.