
Welcome to ESMO 2022
-
The Annual Meeting of the European Society of Medical Oncology (ESMO) was a hybrid meeting, with delegates attending in person for the first time since before the pandemic. ESMO President Solange Peters noted that this made the meeting “feel like a huge success before it has even begun” as the oncology community were able to meet, network and disseminate data in-person in Paris, France. She underlined that, for ESMO, there was never going to be a return to “normal” but was instead progressing forward due to the proactivity that the community has demonstrated in reinventing itself to meet the challenges of our era.

New cancer cases predicted globally by 2030 and 2040
Globally, the ESMO community is already taking action by going the extra mile to comfort patients, to grow as professionals by keeping their knowledge up-to-date, and by raising awareness for cancer prevention. The ESMO community is driven by a shared determination to work towards the best possible outcomes for patients with an inspiring tagline of, ‘Together we can. Together we care.’
Solange Peters then summarised the importance of healthcare sustainability and how our healthcare systems rely on finite resources. She emphasised that a key responsibility of the oncology community should be safeguarding patients’ ability to access high-quality care. ESMO should continue to contribute to the overall sustainability of the healthcare system by nurturing the professional development of oncologists and offering continued support in their day-to-day clinical practice. ESMO’s support and resources should therefore reach every oncology professional.
“As important as doctors’ knowledge and skill is their physical and mental ability to give the best of themselves to patients each day.”
- Solange Peters, ESMO President
Solange Peters then introduced the ESMO 2022 Scientific Co-Chairs, Fabrice André and Charles Swanton. Charles Swanton gave an overview of the impressive proportion of delegates attending ESMO 2022 both in-person and online. He then stated that the purpose of ESMO 2022 is not only a “celebration of getting us all back together”, but also a collaboration between diverse oncology professionals who spend their lives aiming to improving the survival of their patients. ESMO 2022 will focus on “better understanding the disease and treating patients better – from bench to bedside”, which is a matter of time, collaboration, patience and long-term investment. Fabrice André added that current challenges in oncology will be integrated within the ESMO Congress 2022 programme such as early cancer detection and prevention, molecular medicine, and the impact of new drugs assessed in underrepresented patients such as anti-programmed cell death protein-1 (PD1) in the elderly population.

Total participants online and in person
Elements to build a molecular tumour board
Novel therapeutic targets and tumour agnostic treatment
Dr Antoine Italiano, Bordeaux, France, highlighted the change from an empirical and tumour-specific approach to a tumour-agnostic approach with good knowledge of tumour biology and the ability to target specific aberrations. He described the change in clinical trials which are now genotype driven, whereby specific drugs are investigated in a specific molecular subtype.
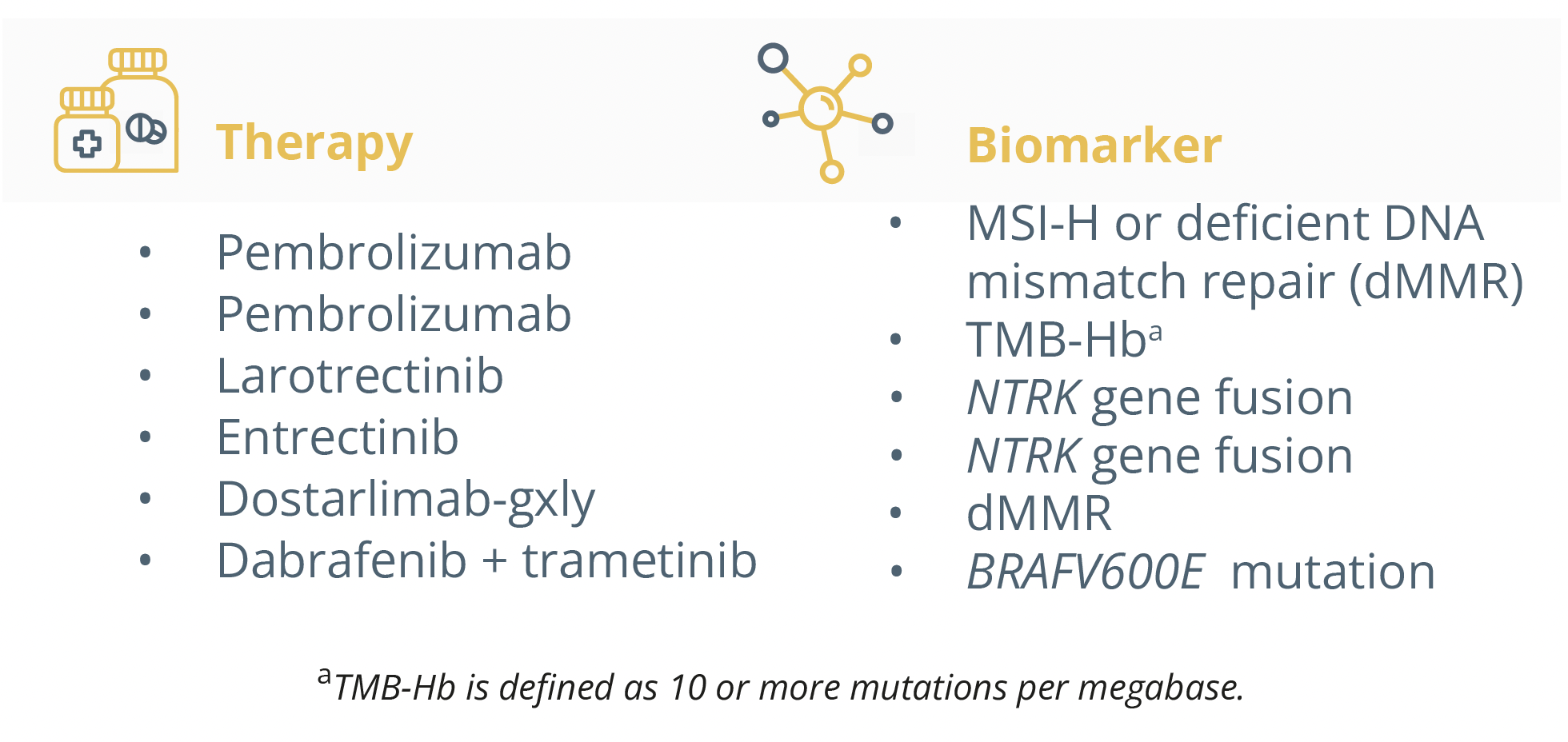
Biomarkers and their associated therapies.
Dr Italiano forecasted an important role for tumour agnostic factors in the new era of personalised healthcare whilst challenging existing diagnostic and value assessment frameworks. Current agnostic drugs that show promise independently of tumour type and histologic status include neurotrophic receptor tyrosine kinase (NTRK) inhibitors. He presented trials (SCOUT NCT02637687 and NAVIGATE NCT02576431) on larotrectinib which is a highly selective inhibitor of NTRK where patients saw clinical benefit regardless of their tumour type. Approaches targeting the RET oncogene have also shown success in the ARROW trial (NCT03037385) as the majority of patients saw clinical benefit regardless of the tumour type. He equally presented recent findings of the ROAR study (NCT02034110) which assessed the efficacy of dabrafenib and trametinib in patients with BRAF mutations. There was a significant objective response rate of over 40% in the majority of histotypes.
He described more current research with FGFR mutations and the broad range of malignancies associated with them. The RAGNAR trial (NCT04083976) assessed the efficacy of erdafitnib in patients with FGFR mutations across a broad range of tumour types. The results showed 117 patients (66%) had a reduction in tumour burden, an objective response rate of 26.4% and clinical benefit rate of 48.9%.
Another study regarding NGR1 fusions, a very rare aberration, was also presented. In the CRESTONE trial (NCT04383210), patients (n=75) with NGR1 fusions received seribantumab and showed 75% of patients responding to treatment, with 75% of these occurring by the first tumour assessment.
He specified that agnostic development can also be used in immuno-oncology as highlighted by the KEYNOTE-158 study (NCT02628067).
“In the field of immuno-oncology, the agnostic development of the drug can also be considered besides genomic aberrations.”
- Dr Antoine Italiano, Bordeaux, France
Dr Italiano equally emphasised the need for funding arrangements to enable better patient access and eligibility for these innovative and personalised medicines.
“We have to negotiate with the regulatory authorities some way to fund the diagnostic test but also fund the innovative drug in order to improve the outcome of the patient.”
- Dr Antoine Italiano, Bordeaux, France
He added that a common understanding and endorsement of tumour-agnostic therapies from both clinical and patient representatives would be crucial in the future to develop the knowledge base and raise awareness for these therapies.
Challenges in the clinical application of molecular tumour boards (MTBs)
Dr Alice Indini, Milan, Italy, began her talk by highlighting the need for MTBs as the management of cancer patients has become increasingly dependent on data from sequencing and molecular analysis, thereby demanding the expertise of clinicians, pathologists and molecular biologists.
“They (MTBs) serve to close the growing gap between our clinical practice and technologic potential in cancer care.”
- Dr Alice Indini, Milan, Italy
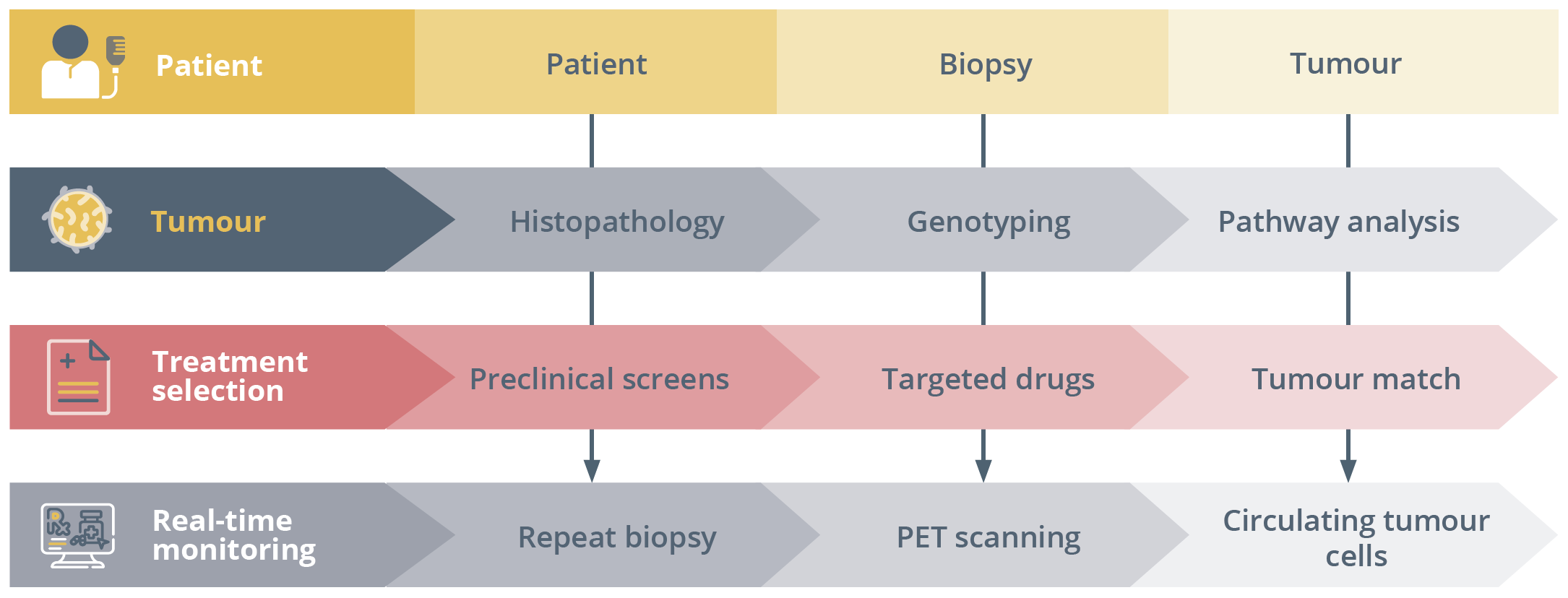
Workflow of the cancer paradigm with genotype-directed cancer therapy.
She specified the potential applications of MTBs for treatment, prevention and early diagnosis as well as for research purposes, underlining that tumour sequencing data could guide individualised anti-cancer treatment. In terms of essential requirements, MTBs require multidisciplinary expertise of a core team, networking between academic and non-academic centres to guarantee access to novel drugs and clinical trials, access to the best technology and finally the implementation of regulatory aspects.
She presented a survey conducted in 39 hospitals in the Netherlands in which the use of MTB analysis was offered to metastatic cancer patients. The survey showed that <50% of hospitals and 5% of non-academic hospitals had access to MTBs, but that patients could have clinical benefit by increased time on treatment and that MTBs could help patient management.
Dr Indini explained the three elements for optimal MTB functioning including global harmonisation in cancer sequencing practices and procedures with the identification of pathogenic mutations, minimal member and operational requirements for an MTB to function adequately and finally an unsolicited findings policy.
She moved on to present the clinical outcomes of an MTB by highlighting a systematic review of 14 studies reporting data on the clinical benefit for patients (n<100) treated with MTB recommendation. Results showed that limited patients received MTB recommendation therapy (11–43%) but the frequency of achieving clinical benefit was good (42–100%). She underlined the lack of randomised trials and studies controlled for non-MTB-directed outcomes.
She also explained the difficulty in translating data into clinical practice due to the complex and heterogeneous molecular profile of advanced tumours. She presented a study showing the real-world data of an MTB where 715 patients were diagnosed with advanced solid tumours but only 429 patients were evaluable for therapy after the MTB. Results showed that 62% of the evaluable patients were matched to one drug recommended by the MTB and that compliance to the MTB recommendations improved progression-free survival (PFS; p=0.008) and overall survival (OS; p=0.036). However, there are still a range of open questions to be resolved.

Open questions regarding MTBs.
She presented the access, consultation, technology, and evidence (ACTE) tool developed to assess the maturity of an organisation’s MTB to identify which areas would benefit from process improvement and standardisation. These areas form 4 pillars and 5 levels with increasing complexity.
“By increasing the complexity of each of these domains we build an adequate tumour board with advanced capabilities.”
- Dr Alice Indini, Milan, Italy
Dr Indini then presented areas of improvement. These included:
- - Guidelines implementation and quality requirements
- - Digital platforms that can help us interpret results and guide treatment decisions
- - Increase access to MTB-recommended medications thanks to increased clinical trial enrolment
- - Real-world data repositories to improve data sharing
- - Prospective validation
She emphasised the need to rely on support systems with complex molecular profiles to understand the best treatment for the patient and presented a modular multi-basket trial to improve personalised medicine (NCT03767075). Results of the trial showed that one third of patients had variants reported as oncogenic mutations which required the interpretation of the functional effect of that variant. The interpretation of cancer gene variants can go from an allele centric interpretation to a tumour context dependent interpretation. There are varying levels of interpretation including the analysis of whether the variant is functionally relevant or neutral. Regarding the study, 65% of the overall variants identified and 72% of patients with unique variants had “unclassified” mutations. From these, only 14% received treatments according to recommendations.
MTBs effectively gather multidisciplinary experience and help decision-making, however there are still some issues notably in sustainability and access, as the majority of patients won’t have access to MTB recommended drugs. Furthermore, prospective data are needed to determine the impact of MTBs on patient outcomes.
Dr Indini concluded by stating that we have clear aims, know which patients and analyses to use but need to improve our knowledge on the functional relevance of the gene variance, turnaround time, accessibility and finally sustainability of MTBs.
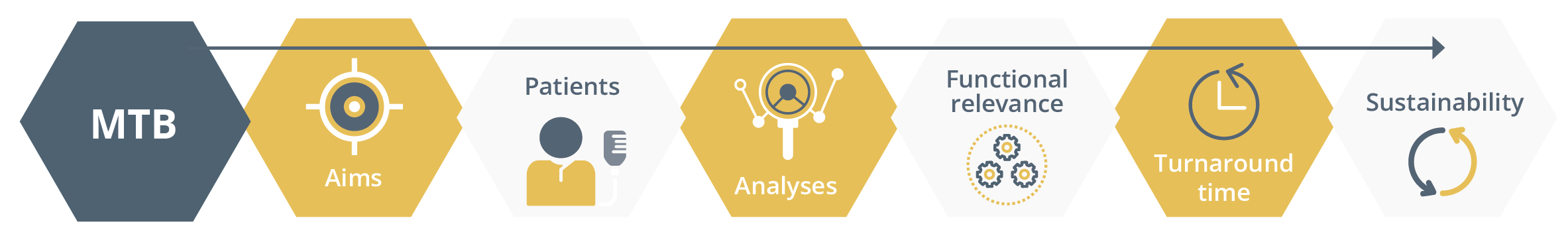
Current understanding and areas of improvement for MTBs
Closing Remarks
Following a successful in-person meeting this year with ~23,000 attendees, ESMO 2023 will continue to disseminate the latest cutting-edge data and provide a unique networking opportunity for oncology professionals in-person in Madrid, Spain, from 20–24 October 2023. Save the date for what will be an equally engaging and successful meeting in 2023.