
Welcome to ESMO 2022
-
The Annual Meeting of the European Society of Medical Oncology (ESMO) was a hybrid meeting, with delegates attending in person for the first time since before the pandemic. ESMO President Solange Peters noted that this made the meeting “feel like a huge success before it has even begun” as the oncology community were able to meet, network and disseminate data in-person in Paris, France. She underlined that, for ESMO, there was never going to be a return to “normal” but was instead progressing forward due to the proactivity that the community has demonstrated in reinventing itself to meet the challenges of our era.

New cancer cases predicted globally by 2030 and 2040
Globally, the ESMO community is already taking action by going the extra mile to comfort patients, to grow as professionals by keeping their knowledge up-to-date, and by raising awareness for cancer prevention. The ESMO community is driven by a shared determination to work towards the best possible outcomes for patients with an inspiring tagline of, ‘Together we can. Together we care.’
Solange Peters then summarised the importance of healthcare sustainability and how our healthcare systems rely on finite resources. She emphasised that a key responsibility of the oncology community should be safeguarding patients’ ability to access high-quality care. ESMO should continue to contribute to the overall sustainability of the healthcare system by nurturing the professional development of oncologists and offering continued support in their day-to-day clinical practice. ESMO’s support and resources should therefore reach every oncology professional.
“As important as doctors’ knowledge and skill is their physical and mental ability to give the best of themselves to patients each day.”
- Solange Peters, ESMO President
Solange Peters then introduced the ESMO 2022 Scientific Co-Chairs, Fabrice André and Charles Swanton. Charles Swanton gave an overview of the impressive proportion of delegates attending ESMO 2022 both in-person and online. He then stated that the purpose of ESMO 2022 is not only a “celebration of getting us all back together”, but also a collaboration between diverse oncology professionals who spend their lives aiming to improving the survival of their patients. ESMO 2022 will focus on “better understanding the disease and treating patients better – from bench to bedside”, which is a matter of time, collaboration, patience and long-term investment. Fabrice André added that current challenges in oncology will be integrated within the ESMO Congress 2022 programme such as early cancer detection and prevention, molecular medicine, and the impact of new drugs assessed in underrepresented patients such as anti-programmed cell death protein-1 (PD1) in the elderly population.

Total participants online and in person
Adjuvant and perioperative therapy for non-colorectal GI cancers: Latest status
Perioperative and adjuvant therapy: gastroesophageal cancer
Gastric and oesophageal cancers
In this educational session, Dr Elizabeth Smyth, Cambridge, UK, described changes on the way for perioperative and adjuvant therapy. She shared the current standards of care in regards to operable gastroesophageal cancer in accordance with the new 2022 ESMO gastric cancer and oesophageal cancer guidelines.
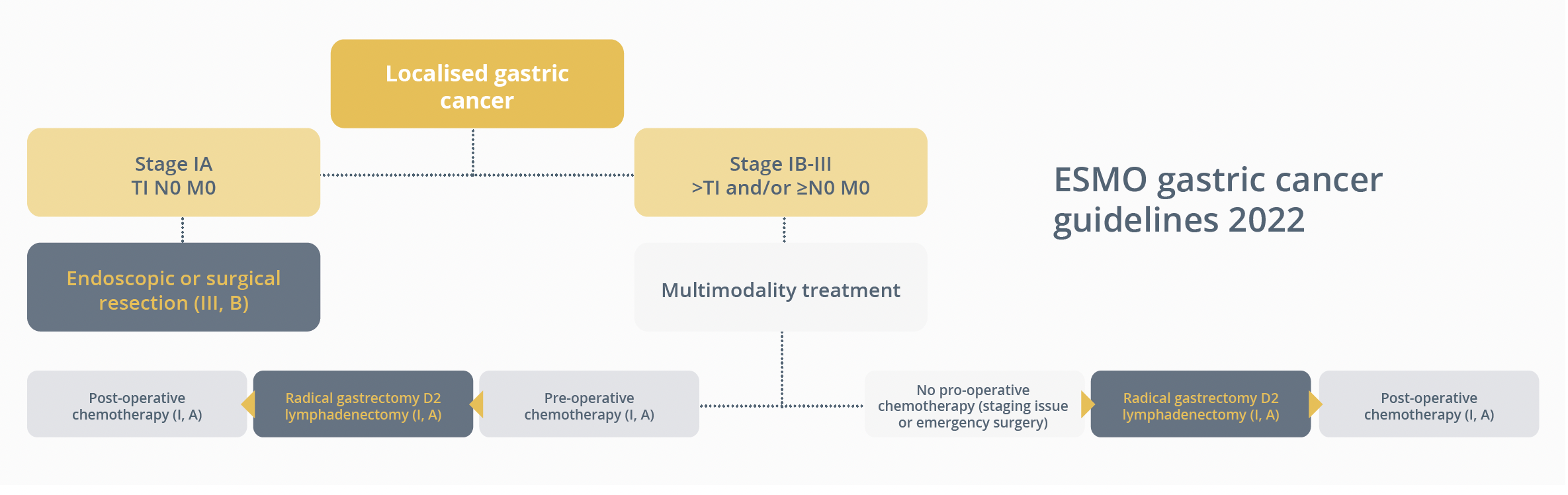
ESMO gastric cancer guidelines 2022
“With the best current treatment that we have, we are curing about 1/3rd to 60% of cancers, so we need to do a little better.”
- Dr Elizabeth Smyth, Cambridge, UK

Therapeutic approaches for gastroesophageal cancer patients.
Dr Smyth predicts treatment regimens to change so that patients currently treated with fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) will have an addition of targeted therapy, immunotherapy or even both. She presented the PETRARCA study (NCT02581462) in which trastuzumab was integrated for the first time in perioperative treatment patterns. Patients with operable gastroesophageal cancer with HER2 positivity treated with FLOT plus trastuzumab/pertuzumab saw a better pathological complete response (pCR) of 41% versus 12% and trend to improved disease-free survival (DFS) than those treated with FLOT alone, respectively. Furthermore, the upcoming EORTC-INNOVATION trial (NCT02205047) will look to tell us the individual benefit of the doublet (perioperative chemotherapy + trastuzumab) versus triplet (perioperative chemotherapy + trastuzumab + pertuzumab) combination of targeted therapy.
Results from studies in advanced HER2+ gastroesophageal cancer indicated the addition of immunotherapy to be key in overall response rates (studies KEYNOTE 811 [NCT03615326] and DESTINY GASTRIC03 [NCT04379596]).
“It is very likely that in future patients who are HER2+ will be treated with a combination of chemotherapy, a HER2 directed therapy and an ICI.”
- Dr Elizabeth Smyth, Cambridge, UK
Current understanding of checkpoint inhibitors and immune sensitivity of patients with adenocarcinoma and oesophageal squamous cell carcinoma (ESCC) was also discussed. Whilst it was shown in a few small trials that anti-PD-1 combined with perioperative chemotherapy improved pCR, toxicity was also increased. Dr Smyth confirmed that bigger trials would be needed to transform the standard of care.
In terms of chemoradiotherapy, Dr Smyth predicts the same approach through the integration of ICI with CROSS, with good results notably for biomarker-selected groups such as high PD-L1 expression as highlighted by the PERFECT study (NCT03087864).
Dr Smyth equally addressed the important question of “do these patients need to have surgery for cure?”. She underlined the concept of improving the pCR to avoid surgery.
“What will be most important will be improving pathological complete response rates in squamous cancers to try and avoid surgery.”
- Dr Elizabeth Smyth, Cambridge, UK
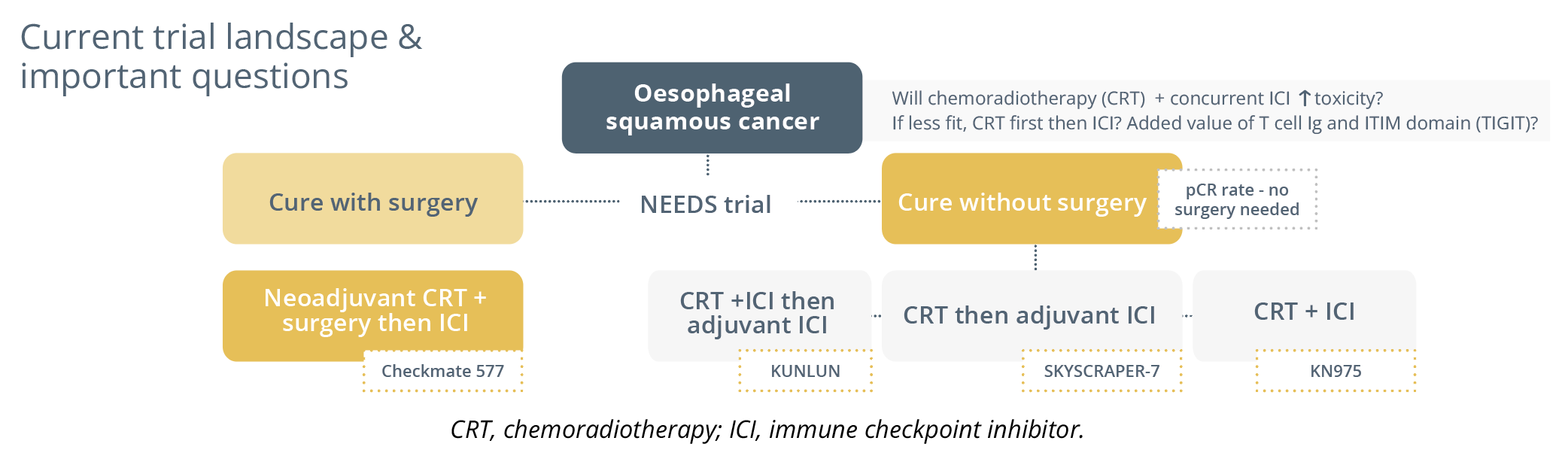
Current trial landscape and important questions
For microsatellite instability (MSI) positive or mismatch repair deficiency (MMRd) tumours, the NEONIPIGA trial (NCT04006262) gave preliminary data highlighting that neoadjuvant and then adjuvant immunotherapy showed promise and that we should now move to an organ-sparing approach saving patients sensitive to immune checkpoint blockade.
She concluded with the potential involvement of ctDNA as a tool to allow us to possibly intensify treatment for some and reduce intensity for others.
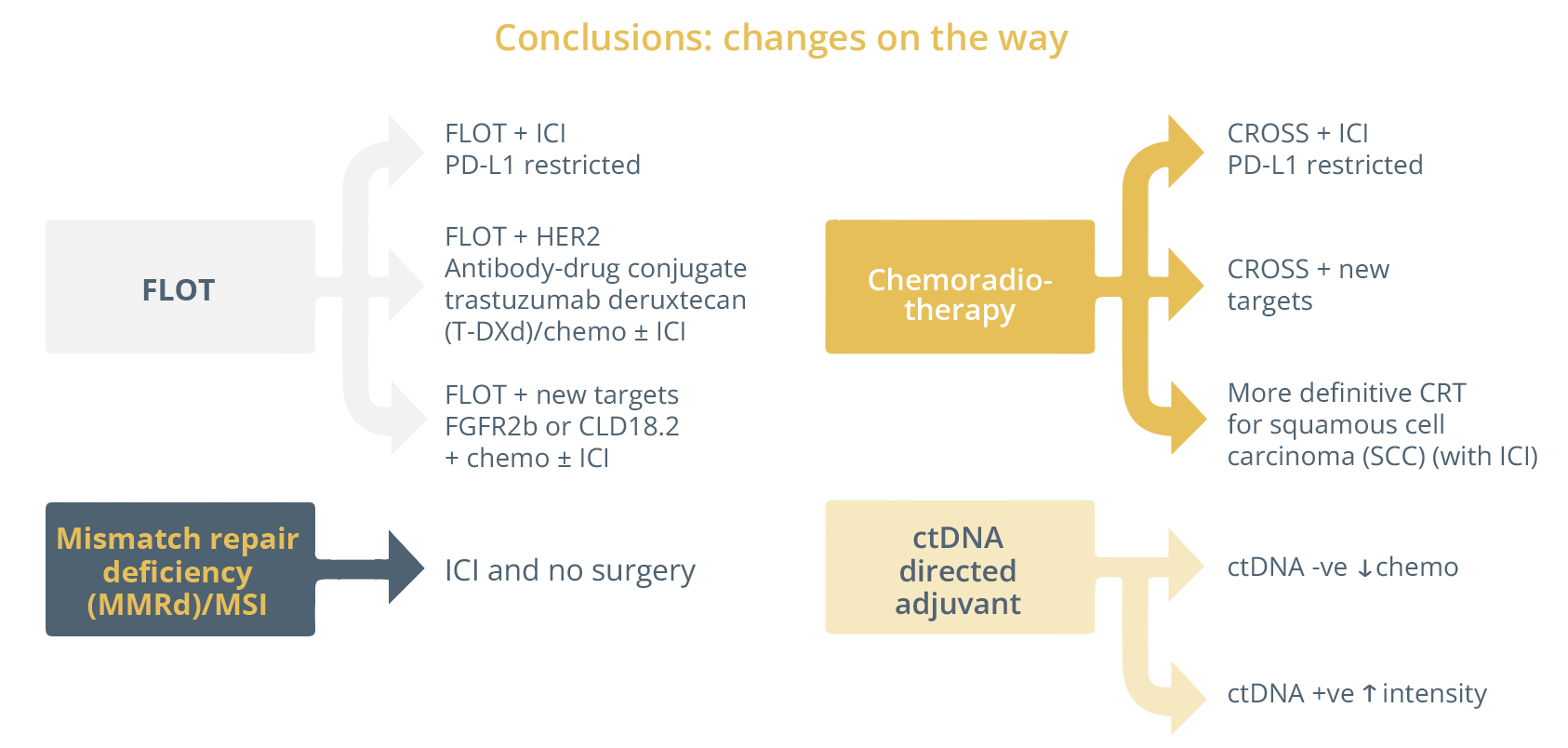
Changes on the way regarding therapeutic approaches for gastroesophageal cancer.
Hepatobiliary cancer
Biliary tract cancer
In her talk, Dr Jennifer Knox, Toronto, Canada, presented the latest status update in the field of biliary cancer (BTC) and hepatic cancer (HCC).
She presented data highlighting the activity within the biliary cancer adjuvant field, but stated that the progress had been inadequate.
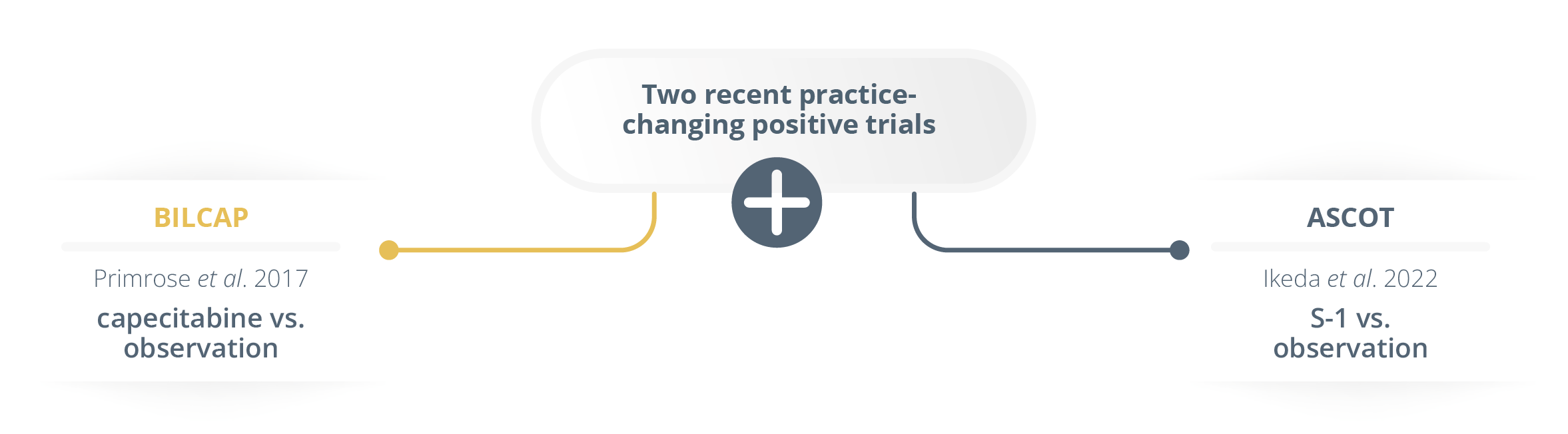
Two recent practice-changing positive trials
As of yet, oral fluoropyrimidines have shown the greatest promise in randomised adjuvant trials. She presented the analysis of the large BILCAP trial (NCT00363584) which yielded practice changing results where patients were randomised to receive capecitabine versus placebo. Whilst the primary endpoint of intention-to-treat (p=0.097) was not met, there was a survival benefit observed within the first 2 years (per protocol OS 53 vs. 36 months for capecitabine versus placebo, respectively, p=0.028). However, 50% of patients in this trial had a local recurrence rate and capecitabine was only shown to delay the recurrence. Dr Knox stressed the need to include molecular subtyping as well as cross trial collaborations in the future.
“Not exactly the slam dunk cure we are thinking about in adjuvant therapy, but probably the delay of recurrence is still a clinically meaningful benefit for patients.”
- Dr Jennifer Knox, Toronto, Canada
For trials limited to extrahepatic sites, the ASCOT trial (UMIN000011688) showed a 3-year OS of 77% versus 68% in favour of S-1 versus observation, respectively.
As of yet, many neoadjuvant studies globally are underway looking at dose intensification around surgery either gemcitabine and cisplatin or gemcitabine/abraxane/platinum (GAP protocol) in the hope of getting a better response rate and a better R0 resection. Studies are assessing the addition of IMI as well as chemoradiation (DEBATE study [NCT04308174], OPTIC study [NCT05514912]).
Dr Knox stressed the need to move past the clinical-pathological features to incorporate biomarkers for a more personalised approach, stating that it is now time for molecular input.
“Moving toward neoadjuvant, peri-operative and downsizing approaches will give more patients the chance for curative intent treatment than we have in the past.”
- Dr Jennifer Knox, Toronto, Canada
Hepatocellular carcinoma
In hepatocellular carcinoma (HCC), progress is also underway with many promising studies evaluating immunotherapy as well as biomarker discovery in the pipeline. Dr Knox added that the analysis of molecular subgroups was also in progress.
“I am optimistic that we are going to see some advancement here.”
- Dr Jennifer Knox, Toronto, Canada
She presented the ongoing adjuvant trials in HCC (Checkmate9Dx [NCT03383458], EMERALD2 [NCT03847428], Imbrave-050 [NCT04102098], and Keynote-927) all evaluating ICIs in higher risk populations as well as downsizing approaches. Dr Knox ended her talk by underlining the importance of a multi-disciplinary approach when tackling all hepatobiliary cancers.
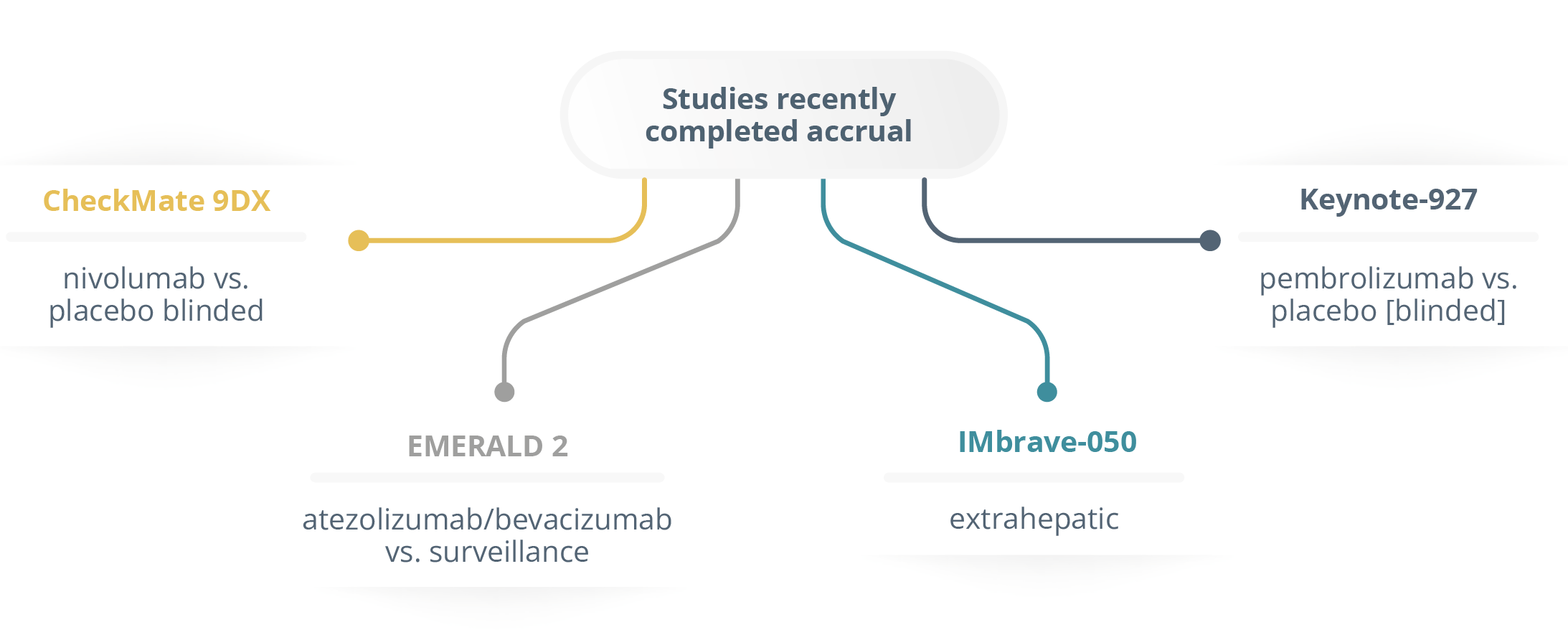
Studies recently completed accrual
“The best outcome for patients occurs when we are open minded in the sequencing of treatment events and always reconsidering localised therapies if they do well on systemic therapy.”
- Dr Jennifer Knox, Toronto, Canada
Perioperative treatment of borderline resectable pancreatic cancer
Dr Thierry Conroy, Vandoeuvre-les-Nancy, France, elaborated on the current treatment approaches for pancreatic cancer (PC). For borderline resectable PC, perioperative treatment is recommended and patients should receive induction therapy as highlighted by the results of the PREOPANC-1 trial (NTR3709) which randomised patients to receive upfront surgery or neoadjuvant chemoradiotherapy. Results showed significant increase in the R0 resection rates (p<0.001) as well as a significant increase in median OS (p=0.029).
ESMO guidelines recommendations 2022
- All patients with localized disease should be evaluated in Multidisciplinary Tumor Board meetings in expert centers (Ill, A).
- Induction therapy is recommended over initial surgery (I, A).
- Patients should be included in clinical trials whenever possible.
- If not feasible, induction chemotherapy (FOLFIRINOX or gemcitabine and nab-paclitaxel), followed by conventional chemoradiation, and then surgery appears to be the best option (Ill, B).
- Following induction therapy, medically fit patients without progression and decrease in CA 19.9 should undergo surgical exploration [Ill, B].
In the adjuvant setting for upfront resectable PC, new data in the JASPAC-01 (UMIN000000655) study showed a large benefit of using S-1 in the Asian population over gemcitabine in terms of survival (p=0.0001). In the Western world the most efficient regimen was mFOLFIRINOX as highlighted by the PRODIGE 24-CCTG PA.6 (NCT01526135) study where patients had significantly better OS (p=0.0009) compared to gemcitabine. Furthermore, Dr Conroy underlined the importance of completing the 6 months of adjuvant chemotherapy as it was a significant positive predictor for OS regardless of the regimen selected in the PRODIGE 24-CCTG PA.6 study (p=0.002).
ESMO guidelines recommendations 2022
- Following resection of PC, adjuvant chemotherapy is strongly recommended.
- mFOLFIRINOX is recommended for patients with resected PC and a good PS.
(ESMO-Magnitude of Clinical Benefit Scale [MCBS] v1.1 score: A). - In patients not candidates for mFOLFIRINOX, (age > 75, Eastern Cooperative Oncology Group Performance Status 2, or contraindication to FOLFIRINOX), gemcitabine/capecitabine is an alternate option (I, B; ESMO-MCBS v1.1 score A).
- Adjuvant gemcitabine alone should be limited to frail patients.
- Adjuvant chemoradiation is not recommended except as part of trials.
Main practical advantages of perioperative treatment
According to concordant meta-analysis, neoadjuvant therapy in resectable PC, as compared to upfront surgery, reduced:
- Resection rate
- Tumours with poor histoprognostic factors
- Tumours with involved lymph nodes
- Postoperative complications and increased R0 resection rate
Interestingly, in the PREOPANC-1 study, the patients with resectable PC did not see an increase in the R0 rate (p=0.54) or median OS (p=0.23), therefore did not see any benefit of neoadjuvant therapy over upfront surgery.
The Japanese trial (Prep-02/JSAP-05) randomising patients to neoadjuvant therapy or surgery and adjuvant therapy was positive but remains unpublished. According to Dr Conroy, neoadjuvant treatment is a logical approach but needs to be validated in large randomised studies. He concluded his talk by highlighting the need for more high quality fully published randomised studies for neoadjuvant therapy in resectable PC and revealed many randomised phase III trials that are ongoing for FOLFIRINOX.
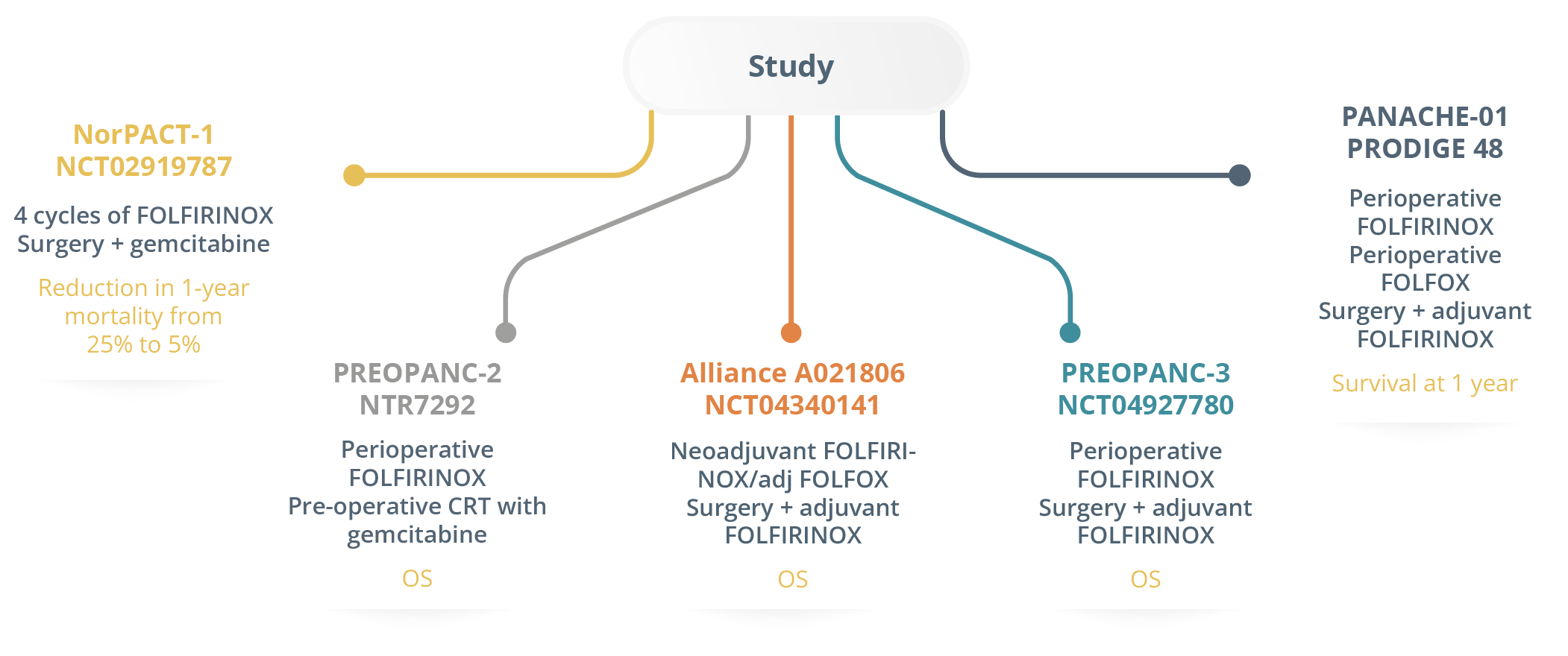
Ongoing randomised phase III trials in resectable PC

The 2022 ESMO guidelines for resectable pancreatic ductal adenocarcinoma and resectable pancreatic cancer
PD-L1 is a good predictive biomarker for anti-PD-1 therapy in oesophagogastric cancer
In this controversy session, chaired by Dr Elizabeth Smyth, Dr Yelena Janjigian, New York, USA and Dr Raghav Sundar, Singapore, Asia, argued the pros and cons of using PD-L1 as a predictive biomarker for anti-PD-1 therapy in oesophagogastric cancer. Dr Smyth opened by underlining the importance of checkpoint inhibitors and why getting PD-L1 is such an important step in the treatment of oesophagogastric cancer.

The importance of PD-L1 in gastroesophageal adenocarcinoma
Whilst anti-PD-1 therapy improves survival and can transform patient lives, the decision to treat or not to treat is a “therapeutic alliance” entered with the patient where the practitioner must decide what the risk-benefit ratio for adverse events associated with these agents is. Other biomarkers have been well established, such as microsatellite instability (MSI), as highlighted by a subgroup analysis of the Checkmate-649 study where patients with MSI-H tumours experienced greater benefit of nivolumab plus chemotherapy than chemotherapy alone. Similarly, patients with PD-L1 Combined Positive Score >5 equally showed higher OS rates and lower risk of death than the randomised population, highlighting the greater magnitude of benefit in biomarker-enriched populations.
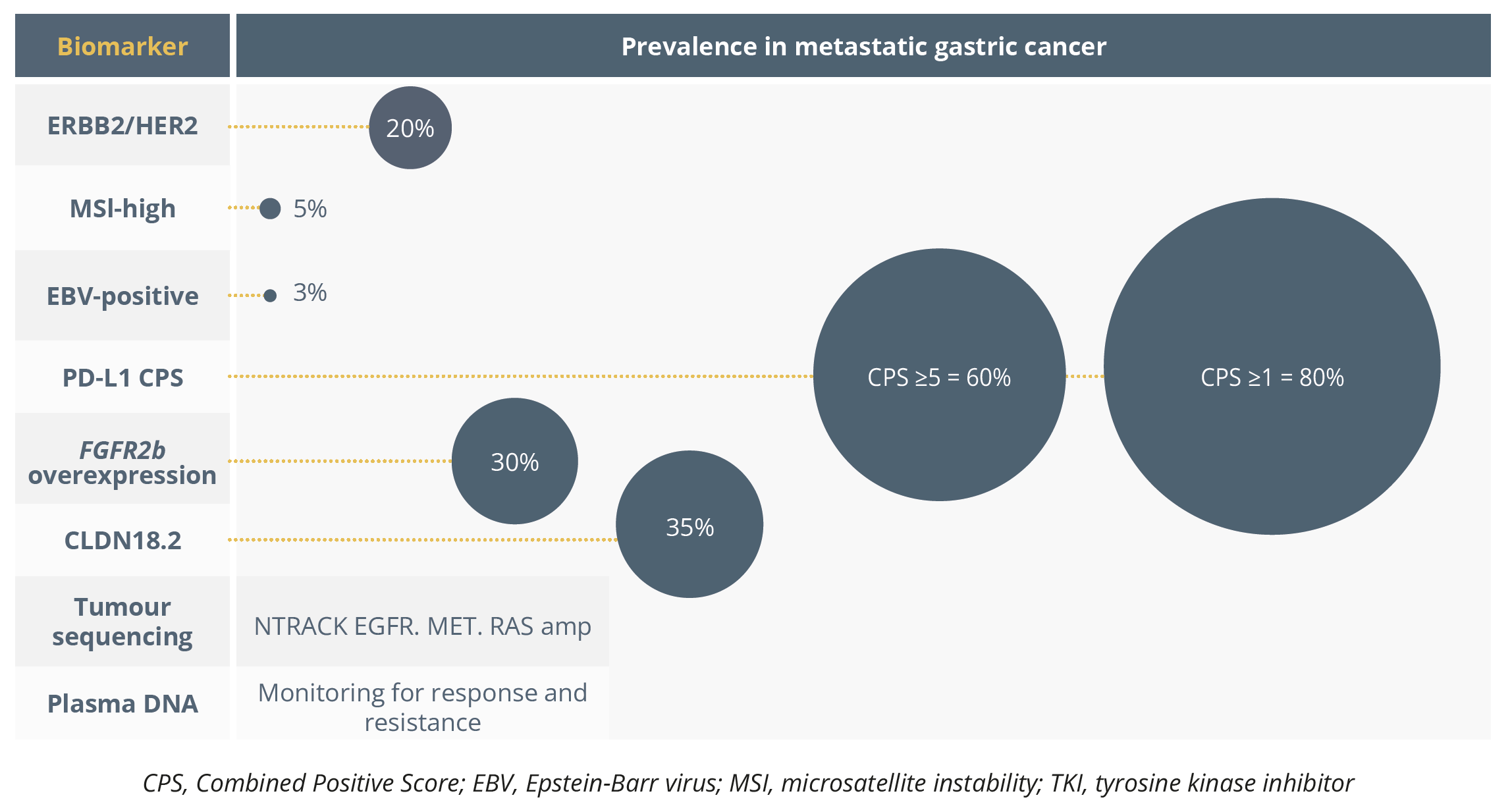
Biomarkers and their prevalence in metastatic gastric cancer
Dr Janjigian emphasised how critical it is to continue to test for HER2, EBV, MSI and PD-L1 and prioritise biomarker selected subgroups in HER2, EGFR, FGFR2a, CLD18.2, and PD-L1 over unselected approaches.
“We need to continue to learn how these (biomarkers) interplay within metastatic disease and potentially early stage disease, so continuing to test is critical.”
- Dr Yelena Janjigian, New York
Dr Raghav Sundar further continued the talk stating that PD-L1 is “but a mediocre biomarker” arguing its lack of analytical validity, clinical validity and clinical utility. He demonstrated PD-L1’s poor ability to discern a benefit for immunotherapy in the Rationale 306 study (NCT03783442) as results showed patients to benefit from tislelizumab plus chemotherapy regardless of PD-L1 status, limiting its clinical utility.
"There is a lack of clarity on how to measure PD-L1 in ESCC.”
- Dr Raghav Sundar, Singapore, Asia
He explained that different trials had different cut-offs, such as study CheckMate 648 (NCT03143153) or ORIENT15 (NCT03748134) that used tumour proportion score (TPS>1) and CPS>10, respectively, thereby limiting its clinical validity. He moved on to discuss its unreliable predictive value for immune checkpoint benefit. In the KEYNOTE 062 study (NCT02494583), the CPS>10 population saw better OS with the single agent pembrolizumab than in combination with chemotherapy. Another point he added was the inability of PD-L1 expression to be measured as a binary value, stating that it is often presented as a continuous variable.
"What is not being presented to us is what is happening in the adjacent subgroups, what is happening CPS 1 to 4, 5 to 10.”
- Dr Raghav Sundar, Singapore, Asia
Variation in assays and CPS cut-offs in trials
- KEYNOTE-590 (pembrolizumab) - Dako 22C3 CPS (CPS ≥1, 10)
- KEYNOTE-062 (pembrolizumab) - Dako 22C3 CPS (CPS 21, 10)
- CheckMate 648 (nivolumab) - Dako 28-8 TPS and CPS (TPS ≥1)
- CheckMate 649 (nivolumab) - Dako 28-8 CPS (CPS ≥1, 5)
He then presented issues with the PD-L1 assay, reiterating the inter-observer variability as well as the varied staining pattern of PD-L1 immunohistochemistry antibodies which can cause a fluctuation in combined positive score (CPS) that determines PD-L1 expression grade. This, in turn, causes doubts as to whether immunotherapy should be used.
Dr Sundar then questioned the reliability of PD-L1 expression due to the heterogeneity of gastric cancer especially since PD-L1 expression has been shown to differ between primary and metastatic tumour site, raising the question of where to biopsy. His last point drew attention to some rare low expressing PD-L1 cases that have long duration of response to immunotherapy.
"The question is not whether PD-L1 is a good biomarker, the question is can we do better than PD-L1 as a biomarker. And for this the answer is a resounding yes.”
- Dr Raghav Sundar, Singapore, Asia
He suggested a composite immunotherapy assay that looks at PD-L1 immunohistochemistry, genomic biomarkers such as MSI and tumour mutational burden and perhaps something novel in the epigenetic or proteomic space to improve the predictive value of our biomarkers for immunotherapy and really improve precision oncology for these patients.
Closing Remarks
Following a successful in-person meeting this year with ~23,000 attendees, ESMO 2023 will continue to disseminate the latest cutting-edge data and provide a unique networking opportunity for oncology professionals in-person in Madrid, Spain, from 20–24 October 2023. Save the date for what will be an equally engaging and successful meeting in 2023.