
The European Alliance of Associations for Rheumatology (EULAR; formerly the European League Against Rheumatism) Congress 2021 was held for the second year running as a virtual meeting because of the global coronavirus disease 2019 (COVID-19) pandemic. As in previous years, its aim was to provide a forum of the highest standard for scientific (both clinical and basic), educational and social exchange between healthcare professionals involved in rheumatology, liaising with patient organisations, in order to achieve progress in the clinical care of people with rheumatic diseases (RMDs).

Prof Iain McInnes, EULAR President
The EULAR e-congress got underway with the traditional opening plenary session, chaired by EULAR President Iain McInnes, who summarised the many accomplishments of EULAR over the past year. These included the launch of the EULAR Virtual Research Centre to support high-quality research in rheumatology across the globe; the efforts of the EULAR advocacy team in working closely with stakeholders across Europe to generate policies that place the needs of patients with RMDs at the fore; and the generation of recommendations, advice and guidance on COVID-19 management. Notably, Prof McInnes highlighted that new and updated EULAR statutes entered into force on 1 January 2021, and to accompany these, EULAR is now the ‘European Alliance of Associations for Rheumatology’. This name change recognises the important role that close collaboration plays in combating RMDs and caring for patients.
“Our adaptation to the pandemic has paved our way to a new era for technology and digital evolution in healthcare and in modern medicine. We have adapted as individuals; we have prevailed, however, as an alliance, and the strides and innovation that we have made this year are a very clear testament to that.”
Anna van der Voort, Netherlands Cancer Institute, Amsterdam, Netherlands
EULAR Recommendations 2021
Robert B. M. Landewė, Amsterdam and Heerlen, The Netherlands, presented the latest updates to the existing recommendations on the management of RMDs in the context of SARS-CoV-2, which were originally published in the Annals of the Rheumatic Diseases in 2020. Currently available literature suggests no evidence that people with RMDs are at greater risk of contracting COVID-19, or have a worse prognosis if they contract COVID-19, than people without RMDs, and risk factors are similar to those of the general population, namely increased age, male sex, high body mass index, and the presence of cardiovascular disease, diabetes and/or chronic lung disease. There does appear to be an increased risk of severe disease and COVID-19-related death associated with glucocorticoid use.
Points to consider on COVID-19 pathophysiology and immunomodulatory therapy use in people with RMDs were reviewed by Alessia Alunno, Perugia, Italy. There is no evidence to support initiation of immunomodulatory therapies in non-hospitalised patients with COVID-19, or those who are hospitalised but do not require oxygen therapy. However, hospitalised patients requiring supplemental oxygen or ventilation should be given systemic glucocorticoids to decrease the risk of mortality, and remdesivir plus baricitinib may also decrease time to recovery and improve clinical status. Hydroxychloroquine should be avoided, because it shows no benefit and may even worsen prognosis.
Javier Rodríguez-Carrio, Oviedo, Spain, outlined preliminary points to consider for the measurement and reporting of interferon (IFN) pathway assays. These are able to predict disease exacerbations, in particular systemic lupus erythematosus flares, progression to clinical disease, and response to IFN-targeted treatment. Despite this, IFN assays are only infrequently used in clinical practice, likely because of the lack of standardisation in research in RMDs, highlighting a need for harmonisation of assay methodology and more robust clinical validation studies.
Raphaela Goldbach-Mansky, Bethesda, MD, USA, and Erkan Demirkaya, London, ON, Canada, presented preliminary recommendations for the diagnosis, treatment and longitudinal monitoring of three groups of novel systemic autoinflammatory diseases, namely interleukin (IL)-1-mediated and type-I interferonopathies, and suspected haemophagocytic lymphohistiocytosis/macrophage activation syndrome. These conditions typically present during childhood, stressing the importance of paediatricians in diagnosis, and are linked to the pathological production of major proinflammatory cytokines, meaning that targeted anti-cytokine therapies can provide effective treatment options that have life-changing effects on patients.
“With the availability of treatment, more of these patients survive into adulthood… so I think there is a true need, not only of diagnosing, but being able to maintain and treat these patients as they... become adults.”
Raphaela Goldbach-Mansky, Bethesda, MD, USA
Synovial tissue biopsy is becoming more widespread in rheumatology, but there is considerable variation in methodology and reporting of results. In recognition of this, Aurelia Najm, Glasgow, UK, presented EULAR guidance on minimal reporting requirements in synovial tissue biopsy clinical applications and research in rheumatology. Three over-arching principles and nine points to consider were developed as part of this research, with the aim of promoting improved understanding, generalisability and repeatability of results from studies involving biopsies.

EULAR guidance on minimal reporting requirements in synovial tissue biopsy clinical applications and research in rheumatology
COVID-19 outcomes in people with rheumatoid arthritis (RA)
The opening plenary abstract session was the most important sessions of this year’s congress, in which the 2021 EULAR abstract award winners presented their research. The first speaker, Jeffrey Sparks, Boston, MA, USA, described results from the COVID-19 Global Rheumatology Alliance (GRA) physician registry on associations between baseline use of biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) and COVID-19 severity in people with RA. From the 15,127 voluntarily reported cases of COVID-19 in the GRA physician registry, patients were identified who also had RA and received b/tsDMARDs at time of COVID-19 onset (n=2869). A variety of b/tsDMARDs were investigated, including abatacept, rituximab, IL-6 inhibitors (tocilizumab, sarilumab), JAK inhibitors (tofacitinib, baricitinib and upadacitinib) and tumour necrosis factor (TNF) inhibitors (infliximab, etanercept, adalimumab, certolizumab pegol and golimumab), which were designated as the reference group. Concomitant use of glucocorticoids and conventional synthetic (cs) DMARDs was allowed. Multivariate ordinal logistic regression analyses showed that use of rituximab or JAK inhibitors for RA was associated with a 4-fold or 2-fold increased risk, respectively, of more severe COVID-19 outcomes versus TNF inhibitor use. In contrast, no consistent associations with COVID-19 severity were observed with abatacept or IL-6 inhibitors. Dr Sparks highlighted that risk mitigation strategies should be a priority in people with RA receiving rituximab or JAK inhibitors who are diagnosed with COVID-19.

ORs (95% CI) for likelihood of poorer COVID-19 outcomes with different drug classes
Synovial tissue biopsy is becoming more widespread in rheumatology, but there is considerable variation in methodology and reporting of results. In recognition of this, Aurelia Najm, Glasgow, UK, presented EULAR guidance on minimal reporting requirements in synovial tissue biopsy clinical applications and research in rheumatology. Three over-arching principles and nine points to consider were developed as part of this research, with the aim of promoting improved understanding, generalisability and repeatability of results from studies involving biopsies.
COVID-19 vaccine safety among people with RMDs
Vaccine hesitancy, often driven by safety concerns, is a barrier to uptake, but can be improved by physician recommendations and advice. Lavanya Rajagopala, Wolverhampton, UK, described the successful education of patients with RMDs about COVID-19 vaccine safety, using an 8-minute, interactive web-based video designed for mobile phone viewing and disseminated by a link sent via SMS text messaging. From 8886 invitations, there were 2358 video views by patients, of whom 664 (28%) completed a short evaluation survey. Prior to watching the video, 52% of patients with RMDs were unsure if COVID-19 vaccination was suitable for them. After viewing the video, 77% (n=509/664) stated that they would be more likely to have the vaccination, and 93% (n=614/660) agreed that the video was a helpful way of sharing information about the COVID-19 vaccine. Dr Rajagopala concluded that this low-cost solution for educating patients about COVID-19 vaccine safety can result in high levels of patient satisfaction, reassurance and self-reported behaviour change. It can easily be updated as new information becomes available and adapted for local language translation, and therefore has potential utility in COVID-19 health promotion in the UK and further afield.
Risk of RA-associated interstitial lung disease (ILD)
Antti Palomäki, Turku and Helsinki, Finland, presented his research on the MUC5B gene promoter variant, rs35705950, and its effect on cumulative long-term incidence of RA-ILD, one of the most common extra-articular manifestations of RA.
The presence of this variant was shown in a 2018 case-control study to significantly increase the likelihood of RA-ILD in people with RA by 3 to 6-fold, compared with the wild type promoter (Juge P, et al. N Eng J Med. 2018;379:2209–19). Data for the study came from the FinnGen project and registry information on medication reimbursement, hospital discharge and cause of death. The aim of FinnGen is to collect genomic information from 500,000 Finnish individuals enrolled in epidemiological cohorts and hospital biobanks, and integrate these with phenotype data from Finnish healthcare registries. Among the 293,972 individuals included in the study, 6869 had RA, of whom 1438 (20.9%) were carriers of the MUC5B gene promoter variant. Carriers had a 16.8% cumulative incidence of RA-ILD by age 80 years, compared with 6.1% in non-carriers (hazard ratio 2.27 [95% CI 1.75, 2.96]). Dr Palomäki stated that because MUC5B was such a common and strong risk factor for RA-ILD in this unselected population over longitudinal follow-up, genetic screening may have the potential for risk stratification of patients with RA-ILD.
Biomarkers of outcomes in treatment-naïve RA
Felice Rivellese, London, UK, described the integrated use of immunohistochemistry, and flow and digital cytometry methods, to characterise distinct immunotypes in peripheral blood and disease tissue from people with early treatment-naïve RA. Using this integrated approach, B-cells were found to be inversely correlated with inflammation and disease activity, T peripheral helper cells emerged as relevant biomarkers of synovial inflammation and potential predictors of clinical outcomes, and T- and B-cell signatures in the peripheral blood were inversely associated with synovial pathotypes.
Passive smoking and the risk of RA
The effect of passive smoking on the risk of incident RA was investigated in the French E3N (Etude Epidémiologique auprès de femmes de l’Éducation Nationale) prospective cohort study conducted by Yann Nguyen, Paris, France and colleagues. The association of environmental factors with chronic diseases is being evaluated in 98,995 women followed since 1990, using self-administered questionnaires. In this cohort, 964 women with RA were identified, of whom 698 were incident RA cases. Passive smoking exposures during childhood and/or adulthood were associated with a significantly increased risk of developing RA. Moreover, age at RA onset appeared to be lower among women exposed to passive smoking during childhood, compared with non-exposed women. Dr Nguyen noted that passive smoking exposure should be limited as much as possible in people at risk of RA to prevent premature onset of the disease.

Passive smoking and the risk of RA
Delivering distance healthcare during the COVID-19 pandemic
This session was devoted to the sharing of experience and best practice from four different specialties regarding distance healthcare during the COVID-19 pandemic. Annette de Thurah, Aarhus, Denmark, presented preliminary findings of a EULAR task force convened in September/October 2020 to provide recommendations on distance healthcare for people with RMDs. Points to consider are still being finalised, but a variety of patient and Health Professionals in Rheumatology (HPR) drivers and barriers were identified.
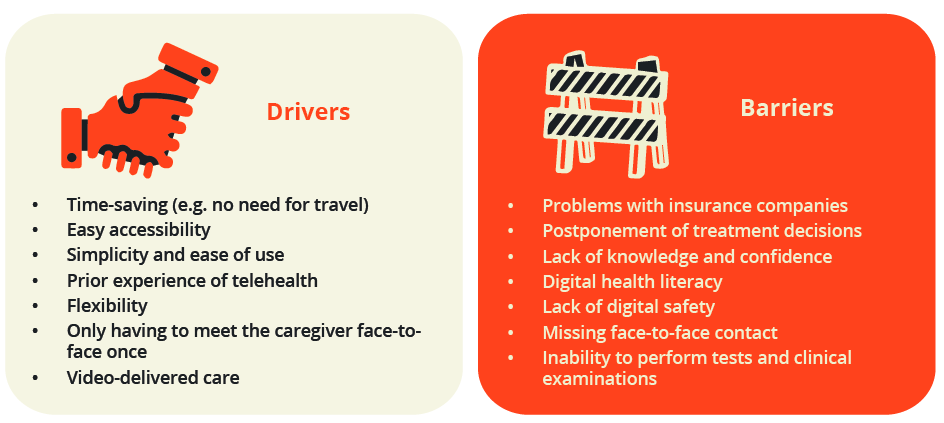
Patient and HPR drivers and barriers
“Healthcare professionals… must… assess and support patients at risk of disparity due to technical inequity, because access to technology and digital literacy skills will have significant influence on the determinants of health for future populations.”
Annette de Thurah, Aarhus, Denmark
Andréa Ascenção Marques, Coimbra, Portugal, affirmed that in this time of videoconferencing, the humble telephone still has prime importance for distance healthcare. During the COVID-19 pandemic, the number of calls by patients approximately doubled compared with before the pandemic, driven by concerns about face-to-face meetings, questions about the safety and appropriateness of ongoing medication use, and the need for emotional support and reassurance, highlighting the value of this traditional method.
Patients’ and physiotherapists’s views on remote physical care delivery during the COVID-19 pandemic were evaluated using surveys and semi-structured interviews by Bas Hilberdink, Leiden, The Netherlands, and colleagues. Data showed that while patients were satisfied with the remote care received and found it beneficial, face-to-face consultations were still preferred. Technical problems, decreased motivation and lack of dedicated equipment or contact with fellow patients were cited as drivers for this preference.
Tracy French, Bristol, UK, explained that the COVID-19 pandemic has generated increased levels of fear and anxiety, social isolation, and reductions in mobility and exercise, and this is magnified in people with RMDs who may be receiving immunosuppressants. Remote consultations make it more difficult to assess physical disease and pick up on non-verbal cues that reveal a patient’s state of mind, meaning these warning signs may be missed; therefore, it is advised that patients should always be asked about their mental health at every remote consultation.
What is new in fatigue in arthritis and systemic rheumatic diseases?
Jette Primdahl, Sønderborg, Denmark, reviewed the latest information on fatigue and its management. Fatigue is a common complaint in people with RMDs, affecting around 35–80% of individuals globally (and this proportion can be higher if multiple RMDs are present), with a recent study reporting prevalences in Denmark of 59%, 77% and 54% in people with RA, psoriatic arthritis or axial spondyloarthritis, respectively. Fatigue is multidimensional and can be caused by a variety of different factors. It is an often misunderstood and under-recognised condition; patients can find it hard to describe their symptoms to work colleagues and often feel that professional support for their condition is lacking, so they must develop their own coping strategies.

Fatigue is multidimensional and can be caused by a variety of factors
One challenge is that fatigue cannot be measured objectively; instead, a multitude of patient-reported outcome measures exist, and these can differ in how they assess fatigue, making comparisons between them difficult. Data on the treatment and management of fatigue come mainly from studies of people with RA. Pharmacological approaches include treatment with biologics, which can lead to a small-to-moderate improvement in fatigue, as well as medications to reduce inflammation and alleviate pain. Depression and other mental health problems should also be addressed. Non-pharmacological management involves supporting the individual to increase their physical activity levels and to learn how to self-manage their condition, and goal-setting and cognitive behavioral therapy can be beneficial in this regard.
“I do hope that all clinicians… will include fatigue in the dialogue with patients, even if we don’t have a quick fix for them.”
Jette Primdahl, Sønderborg, Denmark
New insights into the pathobiology of difficult-to-treat RA (D2T RA)
Nadia Roodenrijs, Utrecht, The Netherlands, summarised recent efforts to formalise the definition of D2T RA and improve its management. The terminology of ‘difficult-to-treat RA’ is the most accurate and encompassing among the myriad descriptions in the literature, and a consensus definition for D2T RA has been developed by a EULAR task force, which describes three mandatory diagnostic characteristics.
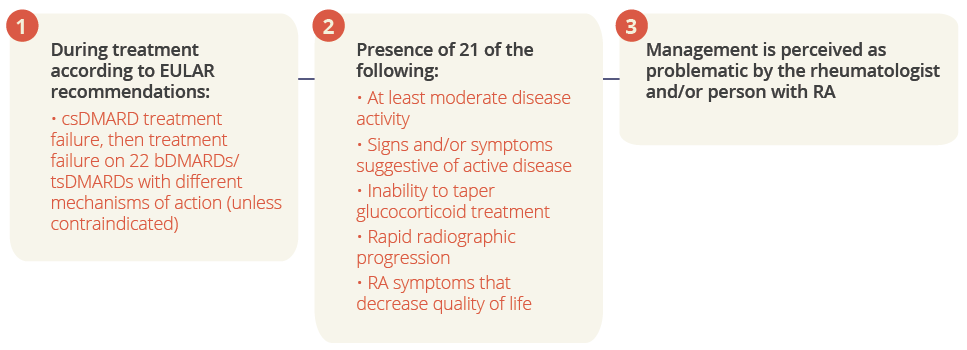
Three mandatory diagnostic characteristics for D2T RA
Costantino Pitzalis, London, UK, described how characterisation of synovial biopsies from people with RA, using histological and molecular signature analysis, enables their classification into different disease pathotypes, which can be used to predict treatment response and refractoriness. csDMARDs and bDMARDs appear to be ineffective in people with a pauci-immune-fibroid synovial pathotype. Tocilizumab appears to be more effective than rituximab in people with a synovial low/absent B-cell lineage signature, while both treatments appear to be equally effective in people with a synovial B-cell rich signature. Because synovial biopsy is well-tolerated, such classification may prove beneficial for the management of RA in the future, pending validation of findings in independent studies.
Elena Nikiphorou, London, UK, highlighted that identification and early targeting of comorbidities is particularly important in D2T RA. Recently, the field has moved toward a more patient-centred approach that also considers comorbidities/multimorbidity, which are common in people with RA from first disease presentation and accumulate over time. Multimorbidity is also associated with increased risk of all-cause mortality. Comorbidities can complicate the assessment of disease activity; for example, evaluation of swollen joint status can be unreliable in people who are overweight or obese, leading to inappropriate treatment decisions being made and suboptimal patient management. Comorbidities can also reduce the effectiveness of treatment and increase adverse drug reactions, negatively impact on treatment goals, disease outcomes and overall prognosis, and are associated with increased healthcare utilisation and costs versus people without these conditions.
“Comorbidities impact on the achievement of treat-to-target goals in RA. For every additional comorbidity, the chance of remission after 1 year was reduced by around 30% and that of low disease activity by around 20%.”
Elena Nikiphorou, London, UK
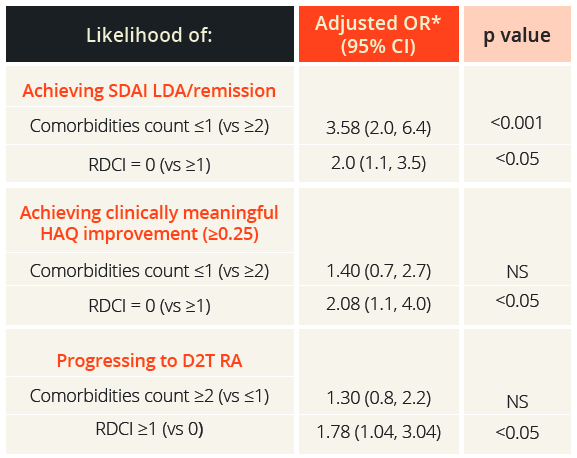
Likelihood of achieving SDAI LDA/remission, clinically meaningful HAQ improvement and progressing to D2T RA
*Adjusted for age, sex, disease duration, seropositivity, number of previous csDMARDs, type of first bDMARD initiated (TNF vs. non-TNF inhibitor), co-administered methotraxate and glucocorticoids (yes/no), baseline simplified disease activity index (SDAI) and health assessment questionnaire (HAQ) and year of therapy start LDA, low disease activity; NS, not significant; RDCI, rheumatic disease comorbidity index
Prodromos Sidiropoulos, Heraklion, Greece, described the findings of a prospective study in 501 people with RA who started their first bDMARD treatment (n=1098 bDMARDs administered) and were recorded in the University of Crete Rheumatology Clinic Registry. A greater number of comorbidities at treatment initiation was an independent predictor of lower 6-month treatment response, as well as progression to D2T RA, once again demonstrating the importance of managing comorbidities in people presenting with RA.
Evolving data on the mechanism of action and safety of JAK inhibitor therapy for RA
Roy Fleischmann, Dallas, TX, USA, reviewed the safety of JAK inhibitors in people with RA, noting that an integrated safety analysis of tofacitinib, baricitinib, upadacitinib and filgotinib showed similar rates of herpes zoster infection, serious infectious episodes, malignancies and major adverse cardiovascular events (MACE) for all treatments. Meta-analysis, registry studies and the clinical development programme of tofacitinib did not reveal a signal for MACE, solid malignancies, venous thromboembolism, pulmonary embolism (PE) or death. In contrast, preliminary analyses from the ORAL Surveillance (NCT02092467) post-marketing safety study, which was mandated by the Food and Drug Administration (FDA), showed that the incidence of PE was significantly increased with tofacitinib 10 mg twice-daily (BID) versus tofacitinib 5 mg BID (the dose approved for use in rheumatic diseases) or TNF inhibitors. Tofacitinib was not non-inferior to TNF inhibitors regarding the occurrence of MACE or malignancies, and the incidences of PE, deep vein thrombosis, arterial thromboembolism or death were comparable between tofacitinib 5 mg BID and TNF inhibitors. Based on these findings, the FDA issued an alert that tofacitinib shows an increased risk of serious cardiac problems, and product labelling was changed to reflect the higher rates of all-cause mortality and thrombosis with the 10 mg BID dose versus the 5 mg BID dose or TNF inhibitors.

Hendrik Schulze-Koops, Munich, Germany, summarised the characteristics of the four JAK family members, the JAK/STAT signalling pathway, and implications for therapy of individual JAK isoform inhibition. JAK molecules form a variety of heterodimeric pairs (and one homodimeric pair, JAK2/JAK2), which then interact with specific combinations of seven different STAT molecules to facilitate cytokine signal transduction; in this way, they mediate inflammation in RA. JAK1, JAK2 and TYK2 are ubiquitously expressed, whereas JAK3 is only present in haematopoietic cells and always in combination with JAK1, meaning that inhibition of either JAK1 or JAK3 can impact on cell signalling via this heterodimer. The selectivity of JAK inhibitors, i.e. their ability at a certain concentration to more potently inhibit a particular JAK isoform versus another, is relative and dose-dependent, and there is the potential to influence multiple JAK isoforms, especially at higher inhibitor concentrations. No JAK inhibitor acts solely on a single JAK isoform. Current data indicate that ‘pan-JAK inhibitors’ and moderately selective JAK inhibitors are comparably effective in RA with similar safety profiles. The concept of JAK selectivity is based on data from in vitro assays, which have limited ability to translate to, and inform, the cellular and clinical aspects of the use of JAK inhibitors to treat RA.
Diego Kyburz, Basel, Switzerland, presented the results of an observational cohort study using data from the Swiss RA registry regarding JAK inhibition in RA, gathered over 10 years of real-life use and including adults with RA who started treatment with tofacitinib (n=793), TNF inhibitors (n=1847) or other mechanism of action bDMARDs (n=1338). TNF inhibitors showed a significantly lower drug retention rate than tofacitinib (hazard ratio 1.29 [95% CI 1.14, 1.47]; p<0.001), with more patients tending to discontinue TNF inhibitors due to ineffectiveness, and tofacitinib due to a greater intolerance to side effects vs. bDMARDs. Stratifying by age showed an increased risk of non-fatal serious infections with tofacitinib vs. bDMARDs for people with RA aged >65 years, compared with those aged <65 years.
People with Arthritis/Rheumatism across Europe (PARE) highlights of EULAR 2021
PARE highlights
Peter Boyd, Dublin, Ireland, provided an overview of the key PARE highlights from EULAR 2021. The COVID-19 pandemic has had a substantial impact on working people with RMDs. Results from European surveys (N=1715 respondents) indicate that 66% of people believed the pandemic had impacted on work or working relationships, and 26% expressed fears that they would be among the first people to lose their job. Thirty-six percent reported benefits from working from home while others perceived this to be an additional burden on top of family life. Numerous opportunities exist for patients to participate in health decision making, engaging as research partners, as patient partners in education, in reviewing lay summaries and making recommendations, and by participating in taskforces, webinars and advisory boards. This involves a considerable amount of time and effort but brings numerous benefits in terms of advancing knowledge and skills, and feeling accepted and valued. The RheumaMap is a research roadmap led by a taskforce of 22 clinicians, scientists and patients that aims to increase the visibility and recognition of RMDs through improvement in disease prevention and earlier diagnosis. It is a living document that identifies current unmet needs and defines priority areas for research.
Closing Remarks
For the second year running, the EULAR congress was a success in its virtual format, providing an excellent forum for lively debate, discussions and exchange of knowledge. EULAR 2022 is scheduled to take place in Copenhagen, Denmark between 1–4 June, and will mark the 75th anniversary of the alliance’s founding.
©Springer Healthcare 2021. This content has been independently selected and developed by Springer Healthcare and licensed by Roche for Medically. The topics covered are based on therapeutic areas specified by Roche. Inclusion or exclusion of any product does not imply its use is either advocated or rejected. Use of trade names is for product identification only and does not imply endorsement. Opinions expressed do not reflect the views of Springer Healthcare. Springer Healthcare assumes no responsibility for any injury or damage to persons or property arising out of, or related to, any use of the material or to any errors or omissions. Please consult the latest prescribing information from the manufacturer for any products mentioned in this material.